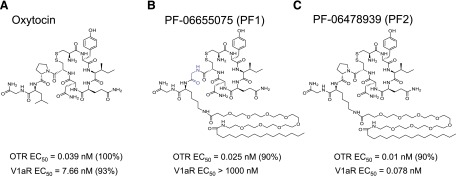
Fig. 1.
Structures of novel long-acting oxytocin analogs. Oxytocin (a) was modified with a substitution of the Leu8 to a Lys appended with a polyethylene glycol space and a palmitoyl group in both PF-06655075 (PF1) (b) and PF-06748939 (PF2) (c) to increase the stability of the molecule. PF1 was further modified with a substitution of Gly for the Pro7 to enhance selectivity for the OT receptor.