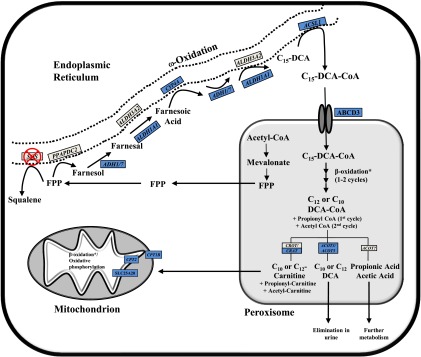
Fig. 8.
Proposed pathway of FPP metabolism after squalene synthase inhibition. Enzymes involved in the cholesterol biosynthetic pathway are localized to different subcellular compartments, with the production of FPP from mevalonate occurring in peroxisomes (Krisans et al., 1994). FPP is used either for the production of squalene by squalene synthase (SQS) or for the synthesis of nonsterol isoprenoids. In the presence of squalene synthase inhibitors (SSI), FPP accumulates and is thought to be metabolized primarily through the farnesol–farnesoic acid–dicarboxylic acid (DCA) pathway (Gonzalez-Pacanowska et al., 1988). Farnesol-derived DCAs are then partially oxidized from the ω-carbon, producing chain-shortened (C12, C10) DCAs that are detectable in urine (Bostedor et al., 1997; Vaidya et al., 1998). The enzymes involved in FPP catabolism have not been fully elucidated; however, based on mRNA gene expression changes observed after SQ1 treatment in the current study, they probably involve components of microsomal, peroxisomal, and mitochondrial oxidation. Shown is a proposed model of FPP catabolism in the presence of squalene synthase inhibitors, which extends on findings from others (Gonzalez-Pacanowska et al., 1988; Bergstrom et al., 1993; Endo et al., 2011; Pant and Kocarek, 2016). FPP can be dephosphorylated by phosphatidic acid phosphatase domain containing 2 (PPAPDC2) to produce farnesol, which is then sequentially oxidized to farnesal and then farnesoic acid. ADH1 and ADH7 were recently identified to catalyze the first oxidation step whereas ALDH3A2 was proposed to catalyze the second (Endo et al., 2011), although ALDH1A1 is another possible candidate based on the expression changes observed in this study. Farnesoic acid is then oxidized from the ω-carbon to produce a C15 dicarboxylic acid. We identified several CYP4A orthologs induced by SQ1, which we propose catalyzes the ω-hydroxylation of farnesoic acid followed by sequential oxidation at the ω-carbon, possibly by the same enzymes involved in farnesol oxidation. The C15-DCA could then be activated by ACSL1 to its CoA derivative for transport into the peroxisome via the dicarboxylic acid transporter ABCD3 (van Roermund et al., 2014), and then undergo one or two rounds of β-oxidation, producing C10 and C12 DCAs as well as propionyl-CoA and acetyl-CoA. The chain-shortened DCAs could either be conjugated with carnitine and exported to the mitochondria for further oxidation or processed by ACOT enzymes, producing free acids that are then eliminated from the cell. For brevity, not all of the enzyme names and/or cofactors are shown for each step. Blue-colored boxes represent orthologous genes that were identified through microarrays to be differentially higher in SQ1-treated compared with untreated controls or to Prav-treated cells (≥1.5-fold). *A number of enzymes involved in peroxisomal and mitochondrial β-oxidation were differentially higher in SQ1-treated compared with control or Prav-treated cells but were excluded for clarity (see Figs. 3 and 4 and text).