FIG 6.
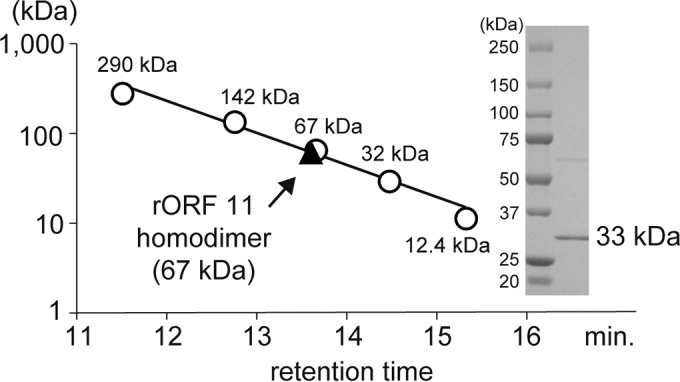
Enzymatic characterization of rOrf11. Purified rOrf11 was subjected to SDS-PAGE. Proteins were stained with CBB R-250. The relative molecular mass of rOrf11 was estimated by gel filtration chromatography (SunSec Diol; 4 mm; 300 by 4.6 mm; ChromaNik Technologies, Osaka, Japan). Glutamate dehydrogenase (290 kDa), lactate dehydrogenase (142 kDa), enolase (67 kDa), myokinase (32 kDa), and cytochrome c (12.4 kDa) were used as the standard molecular masses.
