ABSTRACT
Extracellular proteins play important roles in bacterial interactions with the environmental matrices. In this study, we examined the extracellular proteins from Escherichia coli O157:H7 and O104:H4 by tandem mass spectrometry. We identified 500 and 859 proteins from the growth media of E. coli O157:H7 and O104:H4, respectively, including 371 proteins common to both strains. Among proteins that were considered specific to E. coli O157:H7 or present at higher relative abundances in O157:H7 medium, most (57 of 65) had secretion signal sequences in their encoding genes. Noticeably, the proteins included locus of enterocyte effacement (LEE) virulence factors, proteins required for peptidyl-lipoprotein accumulation, and proteins involved in iron scavenging. In contrast, a much smaller proportion of proteins (37 of 150) that were considered specific to O104:H4 or presented at higher relative abundances in O104:H4 medium had signals targeting them for secretion. These proteins included Shiga toxin 2 subunit B and O104:H4 signature proteins, including AAF/1 major fimbrial subunit and serine protease autotransporters. Most of the abundant proteins from the growth medium of E. coli O104:H4 were annotated as having functions in the cytoplasm. We provide evidence that the extensive presence of cytoplasmic proteins in E. coli O104:H4 growth medium was due to biological processes independent of cell lysis, indicating alternative mechanisms for this potent pathogen releasing cytoplasmic contents into the growth milieu, which could play a role in interaction with the environmental matrices, such as pathogenesis and biofilm formation.
IMPORTANCE In this study, we compared the extracellular proteins from two of the most prominent foodborne pathogenic E. coli organisms that have caused severe outbreaks in the United States and in Europe. E. coli O157:H7 is a well-studied Shiga toxigenic foodborne pathogen of the enterohemorrhagic pathotype that has caused numerous outbreaks associated with various contaminated foods worldwide. E. coli O104:H4 is a newly emerged Shiga toxigenic foodborne pathogen of the enteroaggregative pathotype that gained notoriety for causing one of the most deadly foodborne outbreaks in Europe in 2011. Comparison of proteins in the growth medium revealed significant differences in the compositions of the extracellular proteins for these two pathogens. These differences may provide valuable information regarding the cellular responses of these pathogens to their environment, including cell survival and pathogenesis.
INTRODUCTION
Escherichia coli is a diverse species of bacteria including several pathotypes that cause a variety of diseases in humans. Enterohemorrhagic E. coli (EHEC) is responsible for numerous outbreaks of human enterohemorrhagic disease caused by consumption of contaminated food, including meat and fresh vegetables. The primary reservoirs of the most extensively studied EHEC serotype O157:H7 are cattle and other ruminants (1, 2), but E. coli O157:H7 also is capable of long-term survival in various foods and environmental matrices (3–5).
A recently recognized class of EHEC, represented by Shiga toxigenic enteroaggregative E. coli (EAEC) O104:H4, was responsible for a major outbreak in Germany and other countries in Europe that resulted in nearly 4,000 infections, including 53 deaths (6). Several investigations have compared E. coli O157:H7 and O104:H4 in physiology and pathogenicity and in prevalence in food supplies (7–10). However, no known zoonotic reservoir for E. coli O104:H4 has been identified, and the survival and persistence of E. coli O104:H4 in food and environmental matrices have not been extensively documented. Limited data, however, suggest that E. coli O104:H4 forms biofilms in vitro, and its persistence profiles on fresh produce are comparable to those of O157:H7 (11–14).
Although E. coli O157:H7 and O104:H4 are two of the most prominent Shiga toxigenic foodborne pathogens, they express very different pathogenesis-related factors for interacting with host cells and possibly also with environmental matrices. E. coli O157:H7 expresses the locus of enterocyte effacement (LEE) genes encoding intimin (EaeA), an outer membrane protein, and the translocated intimin receptor (Tir), both important for the formation of attaching and effacing lesions on infected host intestinal epithelial cells (15). E. coli O157:H7 also expresses plasmid-encoded enterohemolysin (EhxA), a pore-forming cytolysin commonly found in serotypes associated with diarrheal disease (16). In contrast, E. coli O104:H4 does not possess these genes and instead expresses aggregative adhesion fimbriae (AAF/1) that enable the bacterial cells to form thick biofilms and “stacked brick” structures on intestinal epithelial cells (17). E. coli O104:H4 also exhibits biofilm formation on leafy green produce and on abiotic surfaces (18). The genomic comparison between E. coli O104:H4 and O157:H7 has revealed that the outbreak strain of E. coli O104:H4 also encodes a heterogeneous set of serine protease autotransporters for virulence and a plasmid-encoded β-lactamase for resistance to β-lactam antibiotics (19, 20).
Extracellular proteins released into the growth milieu play critical roles in cellular interactions with the environmental matrices. They can also be indicative of the expression and turnover of bound cell surface proteins. In this study, we compared E. coli O157:H7 and O104:H4 extracellular proteins released into nutrient-limited M9 minimal growth medium. The mapping of the extracellular proteomes will help improve our understanding of the survival mechanisms for these important foodborne pathogens.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli O157:H7 EDL933 (21, 22) was from the Environmental Microbial and Food Safety Laboratory collection at USDA Agricultural Research Service (ARS), Beltsville, MD. E. coli O104:H4 strain TW16133 (13), isolated from the German sprout outbreak in 2011, was obtained from the Michigan State University EHEC Stock Center. Both strains were grown at 37°C overnight in 400 ml of M9 minimal salts (Becton Dickson, Franklin Lakes, NJ) supplemented with 0.2% glucose and grown to stationary phase (optical density at 600 nm [OD600] = ∼1.3) with shaking. Three separate cultures were grown for each strain.
Protein preparation.
Bacterial cells were separated from the growth medium by centrifugation at 4,000 × g for 20 min, and the supernatant was passed through a low-protein-binding polyethersulfone (PES) filter (0.22 μm) to remove any remaining bacterial cells. Total proteins from the supernatant were concentrated using C8 reverse-phase chromatography (23). Proteins eluted from the column were precipitated in 10% trichloroacetic acid and acetone and washed in cold acetone before being resuspended in 6 M urea–100 mM Tris-HCl, pH 8.5. The protein concentration was determined by a bicinchoninic acid assay (Pierce, Rockford, IL). Recovered proteins (∼100 μg) were reduced in Tris(2-carboxyethyl) phosphine, alkylated with iodoacetamide, and digested overnight at 37°C with Poroszyme immobilized trypsin (Thermo Fisher Scientific, Waltham, MA). The digested peptides were purified by reverse-phase chromatography using SPEC-PLUS PT C18 columns (Varian, Lake Forrest, CA).
Mass spectrometry.
Peptides were analyzed by biphasic liquid chromatography-tandem mass spectrometry, also known as multidimensional protein identification technology (MudPIT) (24–26). Peptides were loaded onto biphasic columns prepared from 365-μm (outer diameter) and 75-μm (inner diameter) fused silica with a 5-μm tip and packed with 9 cm of reverse-phase C18 resin (Aqua, 5 μm; Phenomenex, Torrance, CA) followed by 4 cm of strong cation-exchange resin (Luna, 5 μm; Phenomenex). A new column was used for each sample. A 12-step elution procedure consisting of stepwise increasing concentrations of salt solution followed by increasing gradients of organic mobile phase was performed (27). The solvent flow was 250 nl/min and was controlled with an Accela high-performance liquid chromatography (HPLC) pump (Thermo Fisher Scientific, Waltham, MA) and a T-split junction where 2,100 V was applied (27). Eluent was electrosprayed directly into the orifice of an LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific) controlled by Xcalibur 2.0.7 software (Thermo Fisher Scientific). A parent ion scan was performed in the Orbitrap over the range of 400 to 1,600 m/z at 30,000 resolution, with an automatic gain control (AGC) of 500,000, 500-ms ion injection time, and 1 microscan (μscan). Lock mass was enabled (28). Data-dependent tandem mass spectrometry (MS2) was performed in the linear ion trap with an AGC of 10,000 and 100-ms ion injection times with 1 μscan. MS2 was performed on the 10 most intense MS ions, with minimum signals of 1,000. MS2 spectra were obtained using an isolation width of 2 m/z and a normalized collision energy of 35%. Dynamic exclusion was used with repeat count of 1, a 30-s repeat duration, a list of 500, a list duration of 2 min, and an exclusion mass width of ±0.7 Da. The mass spectrometry data associated with this project can be found at http://massive.ucsd.edu under accession number MSV000079326. The raw data can be downloaded from ftp://massive.ucsd.edu/MSV000079326.
Mascot searching.
MS2 spectrum data files were extracted from the raw data with Bioworks 3.3.1 (Thermo Fisher Scientific) using the following parameters: 600 to 4,500 mass range, 0 group scan, 1 minimum group count, and 5 minimum ion counts. MS2 spectra were searched with Mascot 2.5.1 (29). Search parameters were for tryptic digests with one possible missed cleavage, fixed amino acid modification (+57, C), variable amino acid modifications (+16, M), monoisotopic mass values, ±10-ppm parent ion mass tolerance, ±0.8-Da fragment ion mass tolerance, and #13C = 1 enabled (to compensate for the potential effect of the 13C on peptide mass). Searched databases were downloaded from the National Center for Biotechnology Information (ftp://ftp.ncbi.nlm.nih.gov/genomes/refseq/bacteria). The E. coli O104:H4 database consisted of 4,974 protein records from the bacterial chromosome and 175 protein records from three plasmids for the reference strain 2011C_3493 genome (GenBank accession numbers NC_018658, NC_018659, NC_018660, and NC_018666), and the O157:H7 database consisted of 5,286 protein records from the bacterial chromosome and 99 protein records for one plasmid for the reference strain EDL933 genome (GenBank accession numbers NC_002655 and NC_007414). Both databases were appended with a list of 172 sequences to detect common contaminants, and 23,690 randomized decoy sequences were appended to raise the Mascot Identity score to ensure that peptides were identified by higher-quality peptide-spectrum matches (24, 30, 31).
Mascot output files were processed by PANORAMICS2, a probability-based program that determines the likelihood that peptides are correctly assigned to proteins (32–34). PANORAMICS2 considered peptide matches made by Mascot and for each calculated the probability that a peptide generated an observed spectrum. Analysis was limited to peptides having Mascot Ions score-Identity score differences not less than negative 5. PANORAMICS2 then calculated a probability that a protein or group of proteins (i.e., protein group) was in a sample with respect to the selected database and the observed peptide spectra. The probability that a protein group was not identified by the peptide matches (false-positive rate) is 1 minus the calculated protein probability. Parsimony was achieved by grouping. For any protein group associated with multiple protein records, we chose the first record to represent the group but retained the association to the other records.
Spectral counting was performed, and this procedure has been empirically validated for the relative quantitation of protein abundance (32). A count of 1 was assigned to each peptide for each top-ranked matched spectrum that received a positive peptide probability in PANORAMICS2. The total count for a distinct peptide was based on the total number of qualified spectra, but the total count for a shared peptide was divided by the number of protein groups with which it was shared. These numbers accounting for the identification of all shared and distinct peptides assigned to any one protein group were summed to create a spectral count. Proteins with probabilities of ≥95% comprising at least 1 peptide with at least 1 spectral count were further evaluated. We separately used BLASTP to establish a key to relate all protein sequences of the E. coli O157:H7 genome to those of the O104:H4 genome. There were 4,108 O157:H7 proteins that exhibited ≥90% identity to O104:H4 proteins. This record relationship paired O157:H7 and O104:H4 proteins and allowed for quantization. A normalized spectral abundance factor (NSAF) was generated for each protein by dividing its summed spectral count by its molecular mass and then normalizing that value by the sum of all summed spectral counts divided by molecular mass for this set of proteins (35). For paired proteins, the NSAF values were natural log transformed to create a normal distribution, and a t test was performed to identify significantly different (P < 0.05) ln NSAF values.
Protein annotation and mapping.
The full-length sequences of proteins identified by mass spectrometry were used to search National Center for Biotechnology Information (NCBI) and Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg) databases to determine functional annotation. Secretion signal sequences were predicted using the algorithm at http://effectivedb.org/. KEGG online tools were used for theoretical cellular location and molecular pathway mapping (http://www.genome.jp/kegg).
RESULTS
Detection of proteins from minimal medium.
Proteins from the supernatants were concentrated using C8 reverse-phase chromatography in three pairwise independent replicate experiments with E. coli O157:H7 and O104:H4 grown in M9 minimal media under the same conditions. The average yields of total proteins were 1.4 and 1.6 mg in 400-ml cell cultures, and the OD600 readings were 1.35 and 1.38 for these two strains, respectively, after 16 h of growth. Assuming that 1 OD600 unit is equal to 1 × 109 cells/ml and that E. coli has a dry weight of 2.8 × 10−13 g of cells (36), these yields corresponded to 0.93 and 1.0% of dry cell weight in the growth media. These data indicated that the two strains released similar amounts of proteins into the growth medium.
Using mass spectrometry and assaying medium supernatants from three pairwise trials, we found 677, 792, and 888 proteins for E. coli O157:H7 and 1,144, 1,042, and 1,195 proteins for O104:H4 (data accessible at ftp://massive.ucsd.edu/MSV000079326). To minimize the potential influence of experimental variation, only proteins observed in all three replicates for each strain were considered for further analyses. Hence, there were 500 and 859 proteins common to all three preparations for E. coli O157:H7 and O104:H4, respectively.
Our goal was to compare the proteins from E. coli O157:H7 and O104:H4, but because the genome for E. coli O157:H7 diverges in some protein coding regions compared to that of O104:H4 and because each genome encodes some novel proteins (19), we used Mascot to match E. coli O157:H7 (EDL933) and O104:H4 (TW16133) strain-specific spectra to their respective serotype-specific protein sequence databases (EDL933 and 2011C_3493, respectively). Then, to find commonalities and differences between the data sets, we separately used BLASTP to establish a key that paired the E. coli O157:H7 protein sequences with the O104:H4 protein sequences. Among the 500 proteins common to three trials of E. coli O157:H7, there were 461 that paired with ≥90% identity to O104:H4 records. Then, we examined the 859 proteins common to three trials of E. coli O104:H4 for the 461 pairs and found 371 proteins that were common across all 6 trials using both strains. Because these paired proteins were found in three replicate experiments for each bacterium, we were able to evaluate differential accumulation using spectral counting. Statistics revealed that 118 out of these 371 proteins had significantly different accumulations in the media. Fifty-six had increased abundance in the E. coli O157:H7 growth medium, and 62 had increased abundance in O104:H4 growth medium.
The 371 records were subtracted from the data set of 500 O157:H7 proteins; the remainder comprised 39 unique proteins with <90% identity and 90 proteins with ≥90% identity to O104:H4 proteins not observed across three replicates of O104:H4. Further examination of these 90 proteins revealed that 35 did not have pairs observed in any of the three trials for O104:H4. For the purpose of data presentation and discussion in this article, we refer them as O157:H7-specific proteins, which include O157:H7 unique proteins (<90% identity to O104:H4 records) and those with higher identity with O104:H4 records but which were not detected in the medium of O104:H4 under these experimental conditions. Conversely, proteins unique to O104:H4 and those consistently detected in O104:H4 growth medium but not detected in O157:H7 medium are referred as O104:H4-specific proteins. It need to be recognized that this term does not denote specificity at the genomic or protein expression level. The data for the 74 (39 + 35) O157:H7-specific proteins and the 56 proteins with increased abundance in E. coli O157:H7 growth medium were filtered such that their spectral sums were ≥50, leaving 65 proteins that were highly abundant and reproducibly detected in O157:H7 medium. The spectral sum of 50 was a reasonable cutoff to limit low-level-accumulating proteins (spectral sum maximum = 2,532, minimum = 8, average = 256, and median = 69).
To account for the O104:H4 proteins, we subtracted the 371 proteins that paired with O157:H7; that left 488 proteins that were found across 3 trials with O104:H4. These data were filtered to include only those with <90% identity to O157:H7 and those with pairs not found in any of the three trials for O157:H7 (O104:H4-specific proteins). These proteins and the 62 proteins with increased abundance in O104:H4 growth medium compared to O157:H7 medium were filtered such that their spectral sums were ≥50 (spectral sum maximum = 9303, minimum = 5, average = 164, and median = 52), leaving a remainder of 150 proteins that were highly abundant and reproducibly detected in O104:H4 growth medium.
E. coli O157:H7 extracellular proteins.
Among the set of 65 O157:H7-specific proteins and proteins with significantly increased abundance, 57 were annotated as being extracellular or had signal sequences targeting them for secretion. Hence, most of the proteins detected in the growth medium of E. coli O157:H7 appeared to have been targeted for secretion, as opposed to being leaked into the medium by plasmolysis. Thirty-one of the proteins had ≥90% identity to O104:H4 homologues and accumulated to significantly higher levels than their counterparts (Table 1). The NSAFs for these proteins exhibited a 2-order range of accumulation magnitude, and the most abundant protein was an inhibitor of g-type lysozyme with an NSAF of 8.07E−02. Meanwhile, outer membrane receptors for ferrienterochelins CirA (34-fold) and FepA (23-fold) exhibited the greatest accumulations in fold changes compared to their O104:H4 counterparts (Table 1). In addition, there were 26 unique O157:H7 proteins that had spectral sums of ≥50 and that had secretion signals (Table 2). Most of these proteins were annotated as hypothetical proteins in the NCBI database for E. coli O157:H7 EDL933. Therefore, the KEGG database was used to assign functional annotation. Most were classified as type III secretion system (T3SS) components (37). They included intimin and Tir, proteins that allow O157:H7 cells to attach to intestinal epithelial cells. Intimin and Tir are encoded by the LEE genomic pathogenicity island (38). Several other proteins also expressed from the LEE operon, such as EspA, EspB, Map, and Tir chaperone CesT, were identified in the medium.
TABLE 1.
E. coli O157:H7 proteins with secretion signals and with increased abundance in minimal medium compared to O104:H4
| O157:H7 record | Match to O104:H4 | KEGG and NCBI annotation | Avg NSAFa |
||
|---|---|---|---|---|---|
| O157:H7 | O104:H4 | Ratio | |||
| gi|15802711 | gi|407481290 | CirA; catecholate siderophore receptor | 6.07E−03 | 1.79E−04 | 34 |
| gi|15800298 | gi|407483258 | FepA; outer membrane receptor | 1.92E−03 | 8.39E−05 | 23 |
| gi|15804071 | gi|407479835 | Hypothetical protein Z4941 (predicted Zn-dependent peptidase) | 2.02E−03 | 1.34E−04 | 15 |
| gi|15801644 | gi|407482146 | PqqL; zinc protease | 1.33E−03 | 9.27E−05 | 14 |
| gi|16136752 | gi|407480335 | TolC; outer membrane protein | 8.21E−04 | 8.92E−05 | 9.2 |
| gi|15802168 | gi|407481781 | Tst; thiosulfate/3-mercaptopyruvate sulfurtransferase | 1.64E−03 | 1.84E−04 | 8.9 |
| gi|15802706 | gi|407481295 | MglB; methyl-galactoside transport system substrate-binding protein | 1.76E−03 | 2.47E−04 | 7.1 |
| gi|15799834 | gi|407483677 | FhuA; ferrichrome outer membrane transporter | 6.81E−04 | 9.69E−05 | 7.0 |
| gi|15800571 | gi|407482976 | YbiS; l,d-transpeptidase | 4.52E−03 | 6.49E−04 | 7.0 |
| gi|15801238 | gi|407482519 | PotD; spermidine/putrescine transport system substrate-binding protein | 1.35E−02 | 2.15E−03 | 6.3 |
| gi|15800369 | gi|407483186 | GltI; glutamate/aspartate transport system substrate-binding protein | 2.24E−02 | 3.74E−03 | 6.0 |
| gi|15802857 | gi|407481147 | Amino acid ABC transporter substrate-binding protein | 8.23E−03 | 1.51E−03 | 5.4 |
| gi|15802090 | gi|407481911 | YnhG; l,d-transpeptidase | 1.47E−03 | 2.75E−04 | 5.4 |
| gi|15726736 | gi|407480968 | BamC; outer membrane protein assembly factor | 1.39E−03 | 2.69E−04 | 5.2 |
| gi|15801173 | gi|407482586 | Ycel; polyisoprenoid-binding periplasmic protein | 8.26E−03 | 1.64E−03 | 5.0 |
| gi|15800209 | gi|407483340 | UshA; 5′-nucleotidase/UDP-sugar diphosphatase | 1.67E−03 | 3.54E−04 | 4.7 |
| gi|15803852 | gi|407480056 | Tuf; elongation factor Tu | 1.08E−02 | 2.37E−03 | 4.5 |
| gi|15801838 | gi|407482272 | PppA; oligopeptide transport system substrate-binding protein | 1.39E−03 | 3.11E−04 | 4.5 |
| gi|15800472 | gi|407483096 | ModA; molybdate transport system substrate-binding protein | 9.66E−03 | 2.27E−03 | 4.3 |
| gi|15802055 | gi|407481947 | SlyB; outer membrane lipoprotein | 5.59E−03 | 1.36E−03 | 4.1 |
| gi|15800307 | gi|407483249 | Iron complex transport system substrate-binding protein | 1.28E−03 | 3.26E−04 | 3.9 |
| gi|15804321 | gi|407484536 | PstS; phosphate transport system substrate-binding protein | 2.86E−03 | 7.92E−04 | 3.6 |
| gi|15801009 | gi|407482704 | EfeO; iron uptake system component | 3.39E−02 | 9.88E−03 | 3.4 |
| gi|15800566 | gi|407482981 | OmpX; outer membrane protein X | 1.27E−02 | 3.93E−03 | 3.2 |
| gi|15802788 | gi|407481212 | Glycerophosphoryl diester phosphodiesterase | 2.03E−03 | 6.61E−04 | 3.1 |
| gi|15803968 | gi|407479935 | LivK; branched-chain amino acid transport system substrate-binding protein | 1.18E−02 | 4.67E−03 | 2.5 |
| gi|15801400 | gi|407482486 | Inhibitor of g-type lysozyme | 8.07E−02 | 3.25E−02 | 2.5 |
| gi|16136759 | gi|407481675 | ZnuA; zinc transport system substrate-binding protein | 3.58E−02 | 1.48E−02 | 2.4 |
| gi|15804445 | gi|407484414 | DsbA; thiol:disulfide interchange protein | 1.39E−02 | 6.17E−03 | 2.3 |
| gi|15799845 | gi|407483666 | DegP; serine protease Do | 1.77E−03 | 8.02E−04 | 2.2 |
| gi|15803962 | gi|407479941 | UgpB; glycerol 3-phosphate transport system substrate-binding protein | 9.34E−04 | 6.36E−04 | 1.5 |
A normalized spectral abundance factor (NSAF) was generated for each related protein by dividing its summed spectral count by its molecular mass and then normalizing that value by the sum of all summed spectral counts divided by molecular mass for the set of proteins. The NSAF values are averages from three independent replicate experiments.
TABLE 2.
O157:H7-specific proteins targeted for secretion and accumulating in minimal mediuma
| O157:H7 record | NCBI and KEGG annotation | NSAF |
|---|---|---|
| gi|15801516 | NleG; T3SS effector | 1.31E−01 |
| gi|15804217 | EspA | 1.03E−01 |
| gi|15801940 | NleA; T3SS effector | 4.01E−02 |
| gi|15801929 | NleG8; T3SS effector | 3.50E−02 |
| gi|15801755 | NleG8-like; T3SS effector | 2.94E−02 |
| gi|15804213 | YscF; T3SS effector | 2.83E−02 |
| gi|15804215 | EspB | 2.32E−02 |
| gi|15801928 | IpgB2; BfpT-regulated chaperone | 2.23E−02 |
| gi|15801756 | NleG; T3SS effector | 1.89E−02 |
| gi|15801938 | NleH; T3SS effector | 1.86E−02 |
| gi|15801391 | OmpT; omptin | 1.36E−02 |
| gi|15800491 | LpxR; lipid A 3-O-deacylase | 1.13E−02 |
| gi|15804222 | Tir; translocated intimin receptor | 6.95E−03 |
| gi|15801937 | NleF; T3SS effector | 6.87E−03 |
| gi|15804223 | Map; LEE-encoded effector | 5.90E−03 |
| gi|15804221 | CesT; Tir chaperone | 5.39E−03 |
| gi|15800522 | NleH; T3SS effector | 5.15E−03 |
| gi|15804251 | YjiK; esterase | 4.64E−03 |
| gi|15803145 | EspW; T3SS effector | 3.55E−03 |
| gi|15802410 | EspJ; non-LEE-encoded effector protein | 3.52E−03 |
| gi|15803998 | LolA; outer membrane lipoprotein carrier | 3.31E−03 |
| gi|15803144 | NleG8-like; T3SS effector | 2.29E−03 |
| gi|15803521 | NleE; T3SS effector | 2.23E−03 |
| gi|15804220 | EaeA; intimin | 2.21E−03 |
| gi|15800521 | NleC; T3SS effector | 1.96E−03 |
| gi|15801390 | Protease encoded within prophage | 1.47E−03 |
All proteins in this table have identity of <90% to O104:H4 records and are considered O157:H7 unique proteins.
Using KEGG, we examined the molecular and biological pathways that were represented. The increased accumulation of at least 11 ABC transporters, several bacterial secretion system proteins (TolC and YscF), and two-component signaling system proteins (PstS, DegP, and GltI) implied that the expression of these proteins may have been in response to the minimal medium environmental condition, possibly in response to needs for scavenging nutrients through the transporters (Table 1). It also is possible that this nutrient-poor and stressful environment stimulated effector secretion and LEE-encoded T3SS protein production (Table 2).
E. coli O104:H4 extracellular proteins.
Among the 150 O104:H4-specific proteins and those with increased abundance, only 37 (25%) were annotated as secreted, compared to 88% for O157:H7. Six of these showed increased accumulation in O104:H4 growth medium compared to O157:H7 homologues (Table 3), while the other 31 proteins, including β-lactamase, Shiga toxin 2 subunit B, and several serine protease autotransporters, were not detected in the growth medium of O157:H7 and were considered specific to O104:H4 (Table 4). While β-lactamase confers antibiotic resistance, Shiga toxin 2 subunit B and the serine protease autotransporters are important for E. coli O104:H4 virulence. Subunit B of Shiga toxin 2 allows holotoxin surface attachment and retrograde transport into host cells (39). The O104:H4 AAF/1 major fimbrial subunit and a fimbrial chaperone protein, FimC, were also abundant in the growth medium. These results are consistent with the notion that O104:H4 was primed for host cell surface attachment (40).
TABLE 3.
E. coli O104:H4 proteins targeted for secretion and with increased abundance in minimal medium compared to O157:H7
| O104:H4 record | Match to O157:H7 | NCBI and KEGG annotation | Avg NSAF |
||
|---|---|---|---|---|---|
| O104:H4 | O157:H7 | Ratio | |||
| gi|407482158 | gi|15801655 | OsmC; osmotically inducible protein | 4.36E−03 | 4.23E−04 | 10 |
| gi|407483453 | gi|15800095 | TauA; taurine transport system substrate-binding protein | 5.60E−04 | 1.34E−04 | 4.2 |
| gi|407480519 | gi|15803481 | MetK; S-adenosylmethionine synthetase | 3.52E−03 | 1.02E−03 | 3.5 |
| gi|407481314 | gi|15802681 | OpuC; osmoprotectant transport system substrate-binding protein | 2.46E−03 | 1.13E−03 | 2.2 |
| gi|407484509 | gi|15804351 | RbsB; ribose transport system substrate-binding protein | 1.31E−03 | 6.84E−04 | 1.9 |
| gi|407480839 | gi|15803128 | RP-L19; large-subunit ribosomal protein L19 | 2.25E−03 | 1.35E−03 | 1.7 |
TABLE 4.
E. coli O104:H4-specific proteins targeted for secretion and accumulating in minimal medium
| O104:H4 recorda | NCBI and KEGG annotation | NSAF |
|---|---|---|
| gi|407484719 | β-Lactamase class A CTX-M | 2.26E−02 |
| gi|407479883 | Hypothetical protein O3K_01500 | 1.50E−02 |
| gi|407484873 | Esp; serine protease autotransporter | 9.01E−03 |
| gi|407482751* | StxB; Shiga toxin 2 subunit B | 7.09E−03 |
| gi|407484724 | β-Lactamase class A TEM | 5.24E−03 |
| gi|407481333 | Hypothetical protein O3K_08885 | 4.38E−03 |
| gi|407479888 | CusF; Cu(I)/Ag(I) efflux system periplasmic protein | 2.47E−03 |
| gi|407479899 | PcoC; copper-resistant protein | 2.28E−03 |
| gi|407481442 | Hypothetical protein O3K_09440 | 1.60E−03 |
| gi|407483433* | Hypothetical protein O3K_19565 | 1.50E−03 |
| gi|407480423 | Hypothetical protein O3K_04210 | 1.42E−03 |
| gi|407484167* | 3-Demethylubiquinone-9 3-methyltransferase | 9.82E−04 |
| gi|407482619 | Hypothetical protein O3K_15445 | 8.48E−04 |
| gi|407484687 | EDTA-resistant nuclease | 8.39E−04 |
| gi|407479891 | Hypothetical protein O3K_01540 | 8.36E−04 |
| gi|407482628 | TerD; tellurium resistance protein | 7.42E−04 |
| gi|407481443; gi|407482620 | Hypothetical protein O3K_09445 | 6.26E−04 |
| gi|407480299 | Putrescine 2-oxoglutarate aminotransferase | 6.25E−04 |
| gi|407481503* | HchA; molecular chaperone | 6.04E−04 |
| gi|407484827 | Fimbrial subunit AAF/1 | 6.03E−04 |
| gi|407484114 | Ferric aerobactin receptor | 5.57E−04 |
| gi|407480477; gi|407484128 | Esp; serine protease autotransporter | 5.12E−04 |
| gi|407482275 | Hydrolase | 4.88E−04 |
| gi|407480362* | Oxidoreductase, short-chain dehydrogenase/reductase family protein | 4.11E−04 |
| gi|407482134 | FimC; fimbrial chaperone protein | 3.90E−04 |
| gi|407483057 | NmpC; outer membrane porin protein LC | 3.68E−04 |
| gi|407481484 | FyuA; pesticin/yersiniabactin receptor | 2.74E−04 |
| gi|407479602* | Hypothetical protein O3K_00075 | 2.63E−04 |
| gi|407482682* | Outer membrane receptor for ferrienterochelin and colicins | 1.39E−04 |
| gi|407484105 | Esp; serine protease autotransporter | 1.01E−04 |
| gi|407480464 | Hypothetical protein O3K_04430 | 9.77E−05 |
Asterisks indicate proteins with identity of ≥90% to O157:H7 counterparts but not detected in O157:H7 medium. Records without asterisks are proteins considered unique to O104:H4 (identity of <90% with O157:H7 counterparts).
DISCUSSION
The identification of the proteins that are secreted through the membranes of the cells, either to allow attachment to host cells or abiotic surfaces or to provide a response to environmental stimuli, is important to understanding the biology of pathogenic strains like E. coli O157:H7 and O104:H4. Identifying extracellular proteins has posed challenges in the past because of cellular rupturing which leads to a mixing of cytoplasmic and extracellular proteins. Recently, Tiong et al. evaluated the efficiency of five surface protein extraction methods to reduce contaminating cytosolic components (41), but none of the tested methods provided preparations free of cytosolic proteins. In this study, we grew E. coli O157:H7 and O104:H4 in M9 minimal medium, which allowed concentration and purification of extracellular proteins directly from the supernatant without extra cell manipulation. The results for O157:H7 point to the robustness of the approach: 57 out of 65 of the most abundant proteins detected were annotated as being extracellular or had signal sequences targeting them for secretion.
While most of the O157:H7 specific proteins and those with increased abundance in O157:H7 medium were annotated as secreted proteins, most of the 150 O104:H4-specific proteins and those with increased abundance in O104:H4 medium had annotations consistent with cytoplasmic functions. Hence, the identification of a preponderance of cytosolic proteins for E. coli O104:H4, but not O157:H7, implied that O104:H4 was responding differently to stresses of growing in minimal medium. The reason for this is unclear. The increased secretion of OpuC and OsmC in E. coli O104:H4 is consistent with osmotic stress (Table 3). It is known that prophages are activated by osmotic stress, leading to lysis of host cells (42). However, we did not detect phage capsid or tail proteins among the proteins isolated from the growth medium for E. coli O104:H4 after specifically searching the spectra against a phage protein sequence database. Furthermore, we examined cell viability at the point of harvest by BacLight LIVE/DEAD staining and fluorescence microscopy. The percentages of living cells in both O157:H7 and O104:H4 were high (97.5% for O157:H7 and 99.5% for O104:H4), and E. coli O104:H4 had fewer cells with compromised cell membrane integrity than O157:H7 (Fig. 1). Although it can be argued that this staining did not directly account for lysed cells, we should recognize that cell lysis is generally preceded by a loss of membrane integrity. Therefore, the extent of cell lysis in a culture can be reflected by the prevalence of cells with propidium iodide-permeable membranes (red-stained cells). In Fig. 1, the lower ratio of red-stained cells suggests that less cell lysis occurred, if any, in E. coli O104:H4 than in O157:H7 at the time of harvest. These results suggest that cell lysis was not the major cause of the prevalence of cytosolic proteins in O104:H4 growth medium. Instead, the higher number of cytosolic proteins in O104:H4 growth medium seemed to be due to biological processes independent of cell lysis.
FIG 1.
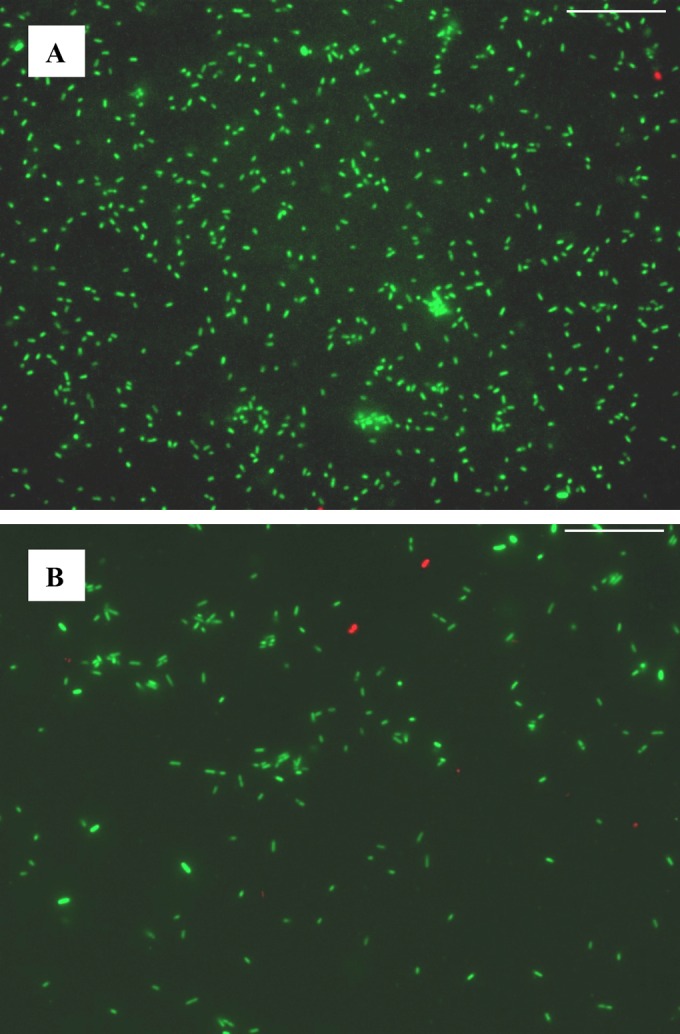
LIVE/DEAD BacLight staining of E. coli O104:H4 (A) and O157:H7 (B) at cell harvest. Membrane-permeative green fluorescent dye SYTO9 stains all cells green, while impermeative red fluorescent dye propiodium only stains the nucleic acids of cells with compromised membrane integrity (dead cells). The calculated cell viability (O104:H4, 99.4%; O157:H7, 97.4%) was based on counting totals of 7,952 and 1,735 cells, respectively. Scale bar: 25 μm.
One possibility is cell leakiness (43–45) for O104:H4. Leaky strains are characterized by their ability to release more alkaline phosphatases into the extracellular medium, by their resistance to colicins and antibiotics, and by their reduced amounts of major outer membrane protein OmpF (46). We did not detect any OmpF in the O104:H4 medium, but we did find alkaline phosphatase, several antibiotic and metal resistance proteins, and outer membrane porin (OmpC). Porins form pore-like structures on the cell surface which allow small molecules to pass to the extracellular medium (47). Alternatively, the presence of higher numbers of cytosolic proteins in O104:H4 could result from outer membrane vesicles (OMVs). Recently, Kunsmann and colleagues showed that O104:H4 shed OMVs containing significant amounts of Shiga toxin in addition to other virulence factors and cytosolic proteins that bind to and are internalized by human intestinal epithelial cells (48). The large amount of Shiga toxin 2B we observed supports this hypothesis, but we have yet to show that OMVs can explain the large number of cystosolic proteins we observed in O104:H4 growth medium.
Although many of the proteins found in the O104:H4 growth medium were cytoplasmic in nature, we were able to identify 37 proteins in the medium with signals targeting them for secretion (Tables 3 and 4), including a serine protease autotransporter (Esp) and serine protease precursors. Serine proteases are the most diverse proteolytic enzymes present in Enterobacteriaceae, and they are implicated in the pathogenicity of many bacterial strains (49). Serine proteases are transported externally through the serine protease autotransporter pathway (SPATE), which is a part of the type V secretion system in Gram-negative bacteria like E. coli (50, 51). These results probably suggest the importance of the SPATE pathway in O104:H4 pathogenesis.
In comparison, E. coli O157:H7 released more iron-scavenging proteins under the growth conditions used in this study. Iron paucity, common to intestinal environments, stimulates EHEC virulence. Under iron stress, bacteria secrete enterochelin, which solubilizes iron and forms the iron-siderophore complex ferrienterochelin (52). We found that CirA, an outer membrane receptor for ferrienterochelin and colicins, was 34 times more abundant in O157:H7 growth medium. It is plausible that the higher abundance of CirA was indicative of O157:H7 responding to the lack of available iron in the minimal medium. Meanwhile, the O157:H7 intimin (EaeA) and receptor (Tir) were highly abundant in the O157:H7 growth medium. Several other LEE-encoded proteins, including EspA, Espj, EspB, CesT, Map, and 11 T3SS proteins, were also identified in O157:H7 growth medium. Tobe et al. (53) showed that the expression of several LEE genes was enhanced for E. coli O157:H7 grown in Dulbecco's modified Eagle medium (DMEM) with very low iron. The responsiveness of LEE gene expression to iron levels was dependent on Fur (ferric uptake regulator). Therefore, our observation is consistent with iron paucity-induced expression of LEE genes for E. coli O157:H7 growing in M9 minimal medium.
To survive outside intestines, E. coli cells must be able to respond to the stresses of nutrient-poor environments. To a certain degree, the minimal medium we used in this study simulated a nutrient-poor environment and likely initiated survival responses. Survival responses could include producing biofilms that allow bacteria to form protective layers. For E. coli O104:H4, we hypothesize that the release of cell contents through porins or OMVs may significantly contribute to the localized accumulation of extracellular proteins and nucleic acids, which promote the initiation of biofilm formation. On the other hand, several outer membrane lipoproteins and proteins involved in lipoprotein-peptidoglycan cross-linking and accumulation, including SylB, LolA, BamC, YbiS, YnhG, TolC, and LpxR, appeared as unique proteins or showed increased abundance in the medium for E. coli O157:H7 (Table 2). Peptidoglycans and lipoproteins are the major components of biofilm matrices (54). These observations seem to suggest that O157:H7 may rely on these proteins to form extracellular matrices for biofilm formation and that this may be initiated by nutrient deficiency.
ACKNOWLEDGMENTS
This research was funded by USDA-ARS and a specific cooperative agreement between USDA-ARS and the University of Maryland, College Park, MD.
We thank Michigan State University EHEC Stock Center for providing E. coli O104:H4 strain TW16133.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
REFERENCES
- 1.Caprioli A, Morabito S, Brugere H, Oswald E. 2005. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet Res 36:289–311. doi: 10.1051/vetres:2005002. [DOI] [PubMed] [Google Scholar]
- 2.Rabatsky-Ehr T, Dingman D, Marcus R, Howard R, Kinney A, Mshar P. 2002. Deer meat as the source for a sporadic case of Escherichia coli O157:H7 infection, Connecticut. Emerg Infect Dis 8:525–527. doi: 10.3201/eid0805.010373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muniesa M, Jofre J, Garcia-Aljaro C, Blanch AR. 2006. Occurrence of Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli in the environment. Environ Sci Technol 40:7141–7149. doi: 10.1021/es060927k. [DOI] [PubMed] [Google Scholar]
- 4.Ogden ID, Hepburn NF, MacRae M, Strachan NJ, Fenlon DR, Rusbridge SM, Pennington TH. 2002. Long-term survival of Escherichia coli O157 on pasture following an outbreak associated with sheep at a scout camp. Lett Appl Microbiol 34:100–104. doi: 10.1046/j.1472-765x.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilks SA, Michels H, Keevil CW. 2005. The survival of Escherichia coli O157 on a range of metal surfaces. Int J Food Microbiol 105:445–454. doi: 10.1016/j.ijfoodmicro.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, Wadl M, Zoufaly A, Jordan S, Kemper MJ, Follin P, Muller L, King LA, Rosner B, Buchholz U, Stark K, Krause G, HUS Investigation Team. 2011. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 365:1771–1780. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 7.Beutin L, Martin A. 2012. Outbreak of Shiga toxin-producing Escherichia coli (STEC) O104:H4 infection in Germany causes a paradigm shift with regard to human pathogenicity of STEC strains. J Food Prot 75:408–418. doi: 10.4315/0362-028X.JFP-11-452. [DOI] [PubMed] [Google Scholar]
- 8.Luchansky JB, Porto-Fett AC, Shoyer BA, Phillips J, Chen V, Eblen DR, Cook LV, Mohr TB, Esteban E, Bauer N. 2013. Fate of Shiga toxin-producing O157:H7 and non-O157:H7 Escherichia coli cells within refrigerated, frozen, or frozen then thawed ground beef patties cooked on a commercial open-flame gas or a clamshell electric grill. J Food Prot 76:1500–1512. doi: 10.4315/0362-028X.JFP-12-432. [DOI] [PubMed] [Google Scholar]
- 9.Luchansky JB, Porto-Fett AC, Shoyer BA, Phillips J, Eblen D, Evans P, Bauer N. 2013. Thermal inactivation of a single strain each of serotype O26:H11, O45:H2, O103:H2, O104:H4, O111:H(−), O121:H19, O145:NM, and O157:H7 cells of Shiga toxin-producing Escherichia coli in wafers of ground beef. J Food Prot 76:1434–1437. doi: 10.4315/0362-028X.JFP-12-429. [DOI] [PubMed] [Google Scholar]
- 10.Porto-Fett AC, Shoyer BA, Thippareddi H, Luchansky JB. 2013. Fate of Escherichia coli O157:H7 in mechanically tenderized beef prime rib following searing, cooking, and holding under commercial conditions. J Food Prot 76:405–412. doi: 10.4315/0362-028X.JFP-12-387. [DOI] [PubMed] [Google Scholar]
- 11.Buvens G, Pierard D. 2012. Infections with verotoxin-producing Escherichia coli O157:H7 and other serotypes, including the outbreak strain O104:H4. Acta Clin Belg 67:7–12. [DOI] [PubMed] [Google Scholar]
- 12.Piérard D, De Greve H, Haesebrouck F, Mainil J. 2012. O157:H7 and O104:H4 Vero/Shiga toxin-producing Escherichia coli outbreaks: respective role of cattle and humans. Vet Res 43:13. doi: 10.1186/1297-9716-43-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Safadi R, Abu-Ali GS, Sloup RE, Rudrik JT, Waters CM, Eaton KA, Manning SD. 2012. Correlation between in vivo biofilm formation and virulence gene expression in Escherichia coli O104:H4. PLoS One 7:e41628. doi: 10.1371/journal.pone.0041628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao Z, Nou X, Luo Y, Wang Q. 2014. Comparison of the growth of Escherichia coli O157: H7 and O104: H4 during sprouting and microgreen production from contaminated radish seeds. Food Microbiol 44:60–63. doi: 10.1016/j.fm.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Elliott SJ, Sperandio V, Giron JA, Shin S, Mellies JL, Wainwright L, Hutcheson SW, McDaniel TK, Kaper JB. 2000. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun 68:6115–6126. doi: 10.1128/IAI.68.11.6115-6126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cookson AL, Bennett J, Thomson-Carter F, Attwood GT. 2007. Molecular subtyping and genetic analysis of the enterohemolysin gene (ehxA) from Shiga toxin-producing Escherichia coli and atypical enteropathogenic E. coli. Appl Environ Microbiol 73:6360–6369. doi: 10.1128/AEM.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denamur E. 2011. The 2011 Shiga toxin-producing Escherichia coli O104:H4 German outbreak: a lesson in genomic plasticity. Clin Microbiol Infect 17:1124–1125. doi: 10.1111/j.1469-0691.2011.03620.x. [DOI] [PubMed] [Google Scholar]
- 18.Nagy A, Xu Y, Bauchan GR, Shelton DR, Nou X. 2016. Aggregative adherence fimbriae I (AAF/I) mediate colonization of fresh produce and abiotic surface by Shiga toxigenic enteroaggregative Escherichia coli O104:H4. Int J Food Microbiol 229:44–51. doi: 10.1016/j.ijfoodmicro.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed SA, Awosika J, Baldwin C, Bishop-Lilly KA, Biswas B, Broomall S, Chain PS, Chertkov O, Chokoshvili O, Coyne S, Davenport K, Detter JC, Dorman W, Erkkila TH, Folster JP, Frey KG, George M, Gleasner C, Henry M, Hill KK, Hubbard K, Insalaco J, Johnson S, Kitzmiller A, Krepps M, Lo CC, Luu T, McNew LA, Minogue T, Munk CA, Osborne B, Patel M, Reitenga KG, Rosenzweig CN, Shea A, Shen X, Strockbine N, Tarr C, Teshima H, van Gieson E, Verratti K, Wolcott M, Xie G, Sozhamannan S, Gibbons HS. 2012. Genomic comparison of Escherichia coli O104:H4 isolates from 2009 and 2011 reveals plasmid, and prophage heterogeneity, including Shiga toxin encoding phage stx2. PLoS One 7:e48228. doi: 10.1371/journal.pone.0048228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perna NT, Plunkett G III, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, Evans PS, Gregor J, Kirkpatrick HA, Posfai G, Hackett J, Klink S, Boutin A, Shao Y, Miller L, Grotbeck EJ, Davis NW, Lim A, Dimalanta ET, Potamousis KD, Apodaca J, Anantharaman TS, Lin J, Yen G, Schwartz DC, Welch RA, Blattner FR. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 21.Karch H, Tarr PI, Bielaszewska M. 2005. Enterohaemorrhagic Escherichia coli in human medicine. Int J Med Microbiol 295:405–418. doi: 10.1016/j.ijmm.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Tarr PI, Gordon CA, Chandler WL. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 23.Niemann GS, Brown RN, Gustin JK, Stufkens A, Shaikh-Kidwai AS, Li J, McDermott JE, Brewer HM, Schepmoes A, Smith RD, Adkins JN, Heffron F. 2011. Discovery of novel secreted virulence factors from Salmonella enterica serovar Typhimurium by proteomic analysis of culture supernatants. Infect Immun 79:33–43. doi: 10.1128/IAI.00771-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper B, Chen R, Garrett WM, Murphy C, Chang C, Tucker ML, Bhagwat AA. 2012. Proteomic pleiotropy of OpgGH, an operon necessary for efficient growth of Salmonella enterica serovar Typhimurium under low-osmotic conditions. J Proteome Res 11:1720–1727. doi: 10.1021/pr200933d. [DOI] [PubMed] [Google Scholar]
- 25.Washburn MP, Wolters D, Yates JR III. 2001. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol 19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 26.Wolters DA, Washburn MP, Yates JR III. 2001. An automated multidimensional protein identification technology for shotgun proteomics. Anal Chem 73:5683–5690. doi: 10.1021/ac010617e. [DOI] [PubMed] [Google Scholar]
- 27.Florens L, Washburn MP. 2006. Proteomic analysis by multidimensional protein identification technology. Methods Mol Biol 328:159–175. [DOI] [PubMed] [Google Scholar]
- 28.Olsen JV, de Godoy LM, Li G, Macek B, Mortensen P, Pesch R, Makarov A, Lange O, Horning S, Mann M. 2005. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol Cell Proteomics 4:2010–2021. doi: 10.1074/mcp.T500030-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551–3567. doi:. [DOI] [PubMed] [Google Scholar]
- 30.Cooper B. 2011. The problem with peptide presumption and low Mascot scoring. J Proteome Res 10:1432–1435. doi: 10.1021/pr101003r. [DOI] [PubMed] [Google Scholar]
- 31.Feng J, Naiman DQ, Cooper B. 2007. Probability-based pattern recognition and statistical framework for randomization: modeling tandem mass spectrum/peptide sequence false match frequencies. Bioinformatics 23:2210–2217. doi: 10.1093/bioinformatics/btm267. [DOI] [PubMed] [Google Scholar]
- 32.Cooper B, Feng J, Garrett WM. 2010. Relative, label-free protein quantitation: spectral counting error statistics from nine replicate MudPIT samples. J Am Soc Mass Spectrom 21:1534–1546. doi: 10.1016/j.jasms.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Feng J, Garrett WM, Naiman DQ, Cooper B. 2009. Correlation of multiple peptide mass spectra for phosphoprotein identification. J Proteome Res 8:5396–5405. doi: 10.1021/pr900596u. [DOI] [PubMed] [Google Scholar]
- 34.Feng J, Naiman DQ, Cooper B. 2007. Probability model for assessing proteins assembled from peptide sequences inferred from tandem mass spectrometry data. Anal Chem 79:3901–3911. doi: 10.1021/ac070202e. [DOI] [PubMed] [Google Scholar]
- 35.Zybailov B, Mosley AL, Sardiu ME, Coleman MK, Florens L, Washburn MP. 2006. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J Proteome Res 5:2339–2347. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert P, Brown MR, Costerton JW. 1987. Inocula for antimicrobial sensitivity testing: a critical review. J Antimicrob Chemother 20:147–154. doi: 10.1093/jac/20.2.147. [DOI] [PubMed] [Google Scholar]
- 37.Tree JJ, Wolfson EB, Wang D, Roe AJ, Gally DL. 2009. Controlling injection: regulation of type III secretion in enterohaemorrhagic Escherichia coli. Trends Microbiol 17:361–370. doi: 10.1016/j.tim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Dean P, Kenny B. 2009. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr Opin Microbiol 12:101–109. doi: 10.1016/j.mib.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukhopadhyay S, Redler B, Linstedt AD. 2013. Shiga toxin-binding site for host cell receptor GPP130 reveals unexpected divergence in toxin-trafficking mechanisms. Mol Biol Cell 24:2311–2318. doi: 10.1091/mbc.E13-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brzuszkiewicz E, Thurmer A, Schuldes J, Leimbach A, Liesegang H, Meyer FD, Boelter J, Petersen H, Gottschalk G, Daniel R. 2011. Genome sequence analyses of two isolates from the recent Escherichia coli outbreak in Germany reveal the emergence of a new pathotype: entero-aggregative-haemorrhagic Escherichia coli (EAHEC). Arch Microbiol 193:883–891. doi: 10.1007/s00203-011-0725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiong HK, Hartson S, Muriana PM. 2015. Comparison of five methods for direct extraction of surface proteins from Listeria monocytogenes for proteomic analysis by orbitrap mass spectrometry. J Microbiol Methods 110:54–60. doi: 10.1016/j.mimet.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Volpi L, Ghisotti D, Sironi G. 1983. Evidence of cell fragility caused by gene kil following lambda induction. Virology 128:166–175. doi: 10.1016/0042-6822(83)90327-6. [DOI] [PubMed] [Google Scholar]
- 43.Chen ZY, Cao J, Xie L, Li XF, Yu ZH, Tong WY. 2014. Construction of leaky strains and extracellular production of exogenous proteins in recombinant Escherichia coli. Microb Biotechnol 7:360–370. doi: 10.1111/1751-7915.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuler TR, Callanan MJ, Klaenhammer TR. 2002. Overexpression of peptidases in Lactococcus and evaluation of their release from leaky cells. J Dairy Sci 85:2438–2450. doi: 10.3168/jds.S0022-0302(02)74326-9. [DOI] [PubMed] [Google Scholar]
- 45.Weigand RA, Rothfield LI. 1976. Genetic and physiological classification of periplasmic-leaky mutants of Salmonella typhimurium. J Bacteriol 125:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazzaroni JC, Portalier RC. 1981. Genetic and biochemical characterization of periplasmic-leaky mutants of Escherichia coli K-12. J Bacteriol 145:1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong KJ, Lee SY. 2002. Excretion of human beta-endorphin into culture medium by using outer membrane protein F as a fusion partner in recombinant Escherichia coli. Appl Environ Microbiol 68:4979–4985. doi: 10.1128/AEM.68.10.4979-4985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunsmann L, Ruter C, Bauwens A, Greune L, Gluder M, Kemper B, Fruth A, Wai SN, He X, Lloubes R, Schmidt MA, Dobrindt U, Mellmann A, Karch H, Bielaszewska M. 2015. Virulence from vesicles: novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain. Sci Rep 5:13252. doi: 10.1038/srep13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Page MJ, Di Cera E. 2008. Serine peptidases: classification, structure and function. Cell Mol Life Sci 65:1220–1236. doi: 10.1007/s00018-008-7565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henderson IR, Cappello R, Nataro JP. 2000. Autotransporter proteins, evolution and redefining protein secretion: response. Trends Microbiol 8:534–535. doi: 10.1016/S0966-842X(00)01884-9. [DOI] [PubMed] [Google Scholar]
- 51.Ruiz-Perez F, Nataro JP. 2014. Bacterial serine proteases secreted by the autotransporter pathway: classification, specificity, and role in virulence. Cell Mol Life Sci 71:745–770. doi: 10.1007/s00018-013-1355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pugsley AP, Reeves P. 1977. Uptake of ferrienterochelin by Escherichia coli: energy dependent stage of uptake. J Bacteriol 130:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tobe T, Yen H, Takahashi H, Kagayama Y, Ogasawara N, Oshima T. 2014. Antisense transcription regulates the expression of the enterohemorrhagic Escherichia coli virulence regulatory gene ler in response to the intracellular iron concentration. PLoS One 9(7):e101582. doi: 10.1371/journal.pone.0101582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godlewska R, Wisniewska K, Pietras Z, Jagusztyn-Krynicka EK. 2009. Peptidoglycan-associated lipoprotein (Pal) of Gram-negative bacteria: function, structure, role in pathogenesis and potential application in immunoprophylaxis. FEMS Microbiol Lett 298:1–11. doi: 10.1111/j.1574-6968.2009.01659.x. [DOI] [PubMed] [Google Scholar]


