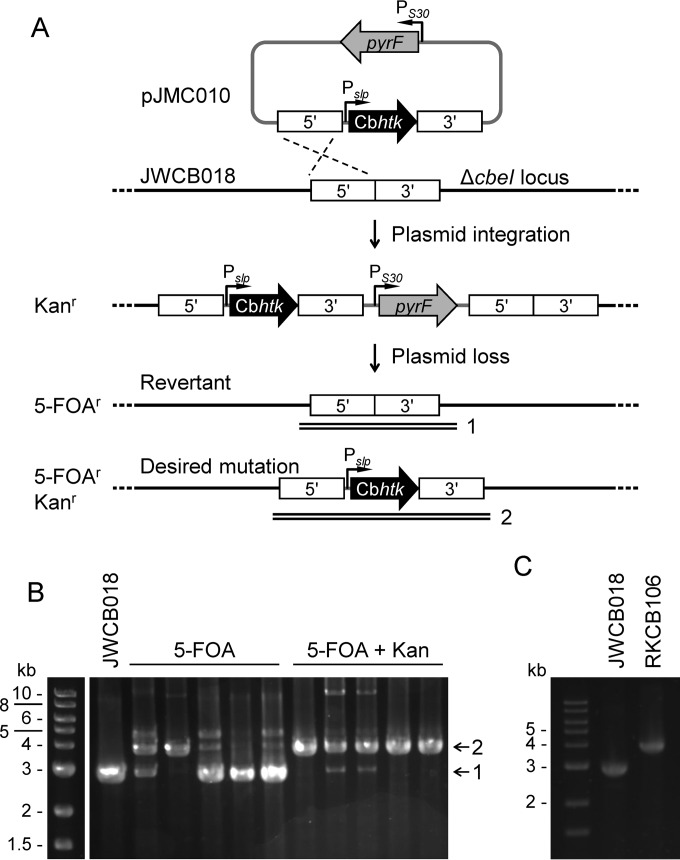
FIG 2.
Direct selection of chromosomal mutations using Cbhtk. (A) Scheme showing selection method for targeted insertion of the PslpCbhtk expression cassette (black) by homologous recombination (dashed lines) into the ΔcbeI locus in C. bescii strain JWCB018 (ΔpyrFA). Kanamycin selected for plasmid integration. To resolve the second crossover event, 5-FOA selected only for plasmid loss, and 5-FOA with kanamycin selected for plasmid loss while maintaining the inserted Cbhtk cassette. Numbered double lines indicate the locations of confirmation PCR products shown in B. (B) PCR screening of isolates counterselected for a second crossover event using solid LOD medium containing 4 mM 5-FOA with or without 10 μg · ml−1 kanamycin. Colonies that resulted from 5-FOA selection were grown in LOD medium with 40 μM uracil, while colonies that resulted from 5-FOAr and Kanr selection were grown in LOD medium with 40 μM uracil and 25 μg · ml−1 kanamycin. The Cbhtk knock-in had an expected amplicon size of 3.9 kb (2) compared to 2.9 kb (1), which was the expected size for the JWCB018 strain. (C) PCR confirmation of PslpCbhtk insertion at the ΔcbeI locus in RKCB106 compared to parent strain JWCB018. PCR products shown in B and C were amplified with primers outside the cbeI flanking regions used for homologous recombination.