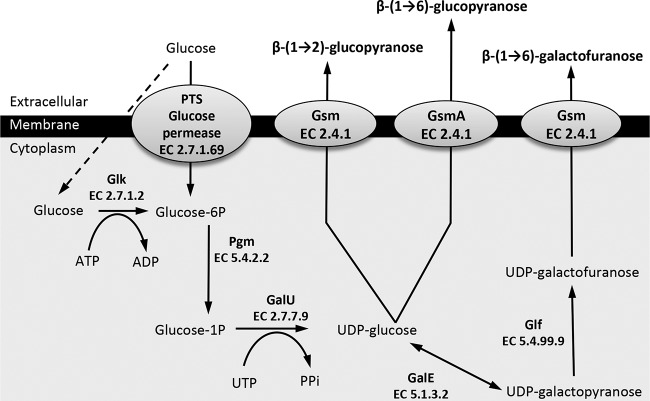
FIG 1.
Predicted biosynthetic pathways of β-(1→2)-glucopyranose, β-(1→6)-glucopyranose, and β-(1→6)-galactofuranose polysaccharides in mycoplasma species of the M. mycoides cluster and M. agalactiae. Glucose uptake and activation into glucose-6-phosphate are mediated by the phosphotransferase system (PTS)-glucose permease or by a two-step process that includes nonspecific permeases (dashed arrow) and a glucokinase (Glk) for phosphorylation. The glucose-6-phosphate is then isomerized by a phosphoglucomutase (Pgm) into glucose-1-phosphate, which in turn is transformed in UDP-glucose by a glucose-1-phosphate uridylyltransferase (GalU). The UDP-glucose is used either directly by a glycosyltransferase with synthase activity (Gsm; glycan synthase of mollicutes) to build and export a β-(1→2)-glucopyranose or a β-(1→6)-glucopyranose polymer [common name, β-(1→2)-glucan or β-(1→6)-glucan, respectively] or further transformed into UDP-galactofuranose by the successive action of an UDP-glucose 4-epimerase (GalE) and an UDP-galactofuranose mutase (Glf). The last-named UDP-sugar monomer is then used by another specific synthase and polymerized into galactan, the common name for the β-(1→6)-galactofuranose polymer. Each enzyme is also designated by its EC number.