FIG 2.
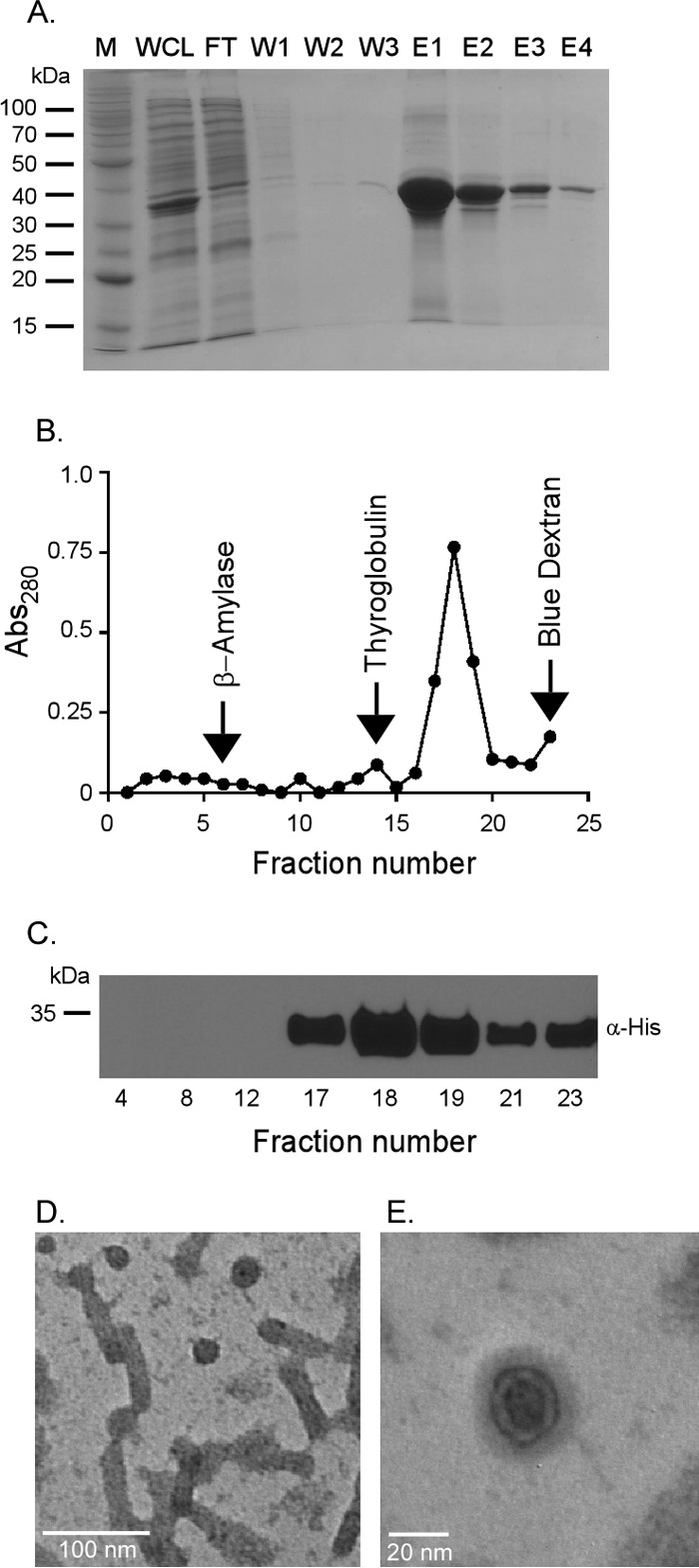
Rv2744c forms higher-order homo-oligomeric structures in vitro. (A) Rv2744c-His was purified via Ni affinity chromatography. Protein fractions were resolved by SDS-PAGE to confirm purity (M, molecular mass marker; WCL, whole-cell lysate; FT, flowthrough; W1 to W3, washes 1 to 3; E1 to E4, elutions 1 to 4). (B) Purified Rv2744c-His was resolved on a continuous 10 to 50% sucrose gradient by ultracentrifugation for 16 h at 4°C and 100,000 × g. Fractions were removed from the tops of the gradient tubes, and the absorbance at 280 nm (Abs280) was quantified via spectrophotometer to determine protein abundance. Protein standards were also separated in parallel and included β-amylase (200 kDa), thyroglobulin (669 kDa), and blue dextran (2,000 kDa). (C) Fractions containing protein were resolved by SDS-PAGE and probed by Western blotting using mouse anti-His antibody in order to confirm the presence of Rv2744c-His. Only selected fractions are shown. (D and E) Purified Rv2744c isolated from fraction 17 of the sucrose gradient was examined via negative-stain electron microscopy at a magnification of ×100,000 (D) or ×300,000 (E). Regular organized structures of small spheroids and tubular structures with similar diameters were observed.
