ABSTRACT
The intrinsic resistance of Enterococcus faecium to ceftriaxone and cefepime (here referred to as “cephalosporins”) is reliant on the presence of class A penicillin-binding proteins (Pbps) PbpF and PonA. Mutants lacking these Pbps exhibit cephalosporin susceptibility that is reversible by exposure to penicillin and by selection on cephalosporin-containing medium. We selected two cephalosporin-resistant mutants (Cro1 and Cro2) of class A Pbp-deficient E. faecium CV598. Genome analysis revealed changes in the serine-threonine kinase Stk in Cro1 and a truncation in the associated phosphatase StpA in Cro2 whose respective involvements in resistance were confirmed in separate complementation experiments. In an additional effort to identify proteins linked to cephalosporin resistance, we performed tandem affinity purification using Pbp5 as bait in penicillin-exposed E. faecium; these experiments yielded a protein designated Pbp5-associated protein (P5AP). Transcription of the P5AP gene was increased after exposure to penicillin in wild-type strains and in Cro2 and suppressed in Cro2 complemented with the wild-type stpA. Transformation of class A Pbp-deficient strains with the plasmid-carried P5AP gene conferred cephalosporin resistance. These data suggest that Pbp5-associated cephalosporin resistance in E. faecium devoid of typical class A Pbps is related to the presence of P5AP, whose expression is influenced by the activity of the serine-threonine phosphatase/kinase system.
IMPORTANCE
β-Lactam antibiotics remain our most effective therapies against susceptible Gram-positive bacteria. The intrinsic resistance of Enterococcus faecium to β-lactams, particularly to cephalosporins, therefore represents a major limitation of therapy. Although the primary mechanism of resistance to β-lactams in E. faecium is the presence of low-affinity monofunctional transpeptidase (class B) penicillin-binding protein Pbp5, the interaction of Pbp5 with other proteins is fundamental to maintain a resistant phenotype. The present work identifies a novel, previously uncharacterized, protein that interacts with Pbp5, whose expression increases in conjunction with stimuli that increase resistance to cephalosporins, and that confers increased resistance to cephalosporins when overexpressed. P5AP may represent a promising new target, inhibition of which could restore cephalosporin susceptibility to E. faecium.
INTRODUCTION
Enterococcus faecalis and Enterococcus faecium differ from the closely related streptococci by virtue of their intrinsic resistance to cephalosporin antibiotics and their reduced susceptibility to penicillins. This resistance has been attributed to the expression of low-affinity class B penicillin-binding protein (Pbp) Pbp5 (1), a transpeptidase that must work in coordination with class A Pbp glycosyltransferases to be able to synthesize peptidoglycan in the presence of cephalosporin antibiotics (2). E. faecium strains in which Pbp5 has been deleted are susceptible to β-lactam antibiotics, including cephalosporins (3). Several other loci are involved in cephalosporin resistance, including two of the three identified class A Pbps (PonA and PbpF) (2, 4), the CroRS system (in E. faecalis) (5), the eukaryote-like kinase/phosphatase systems (Stk/StpA in E. faecium and IreK/IreP in E. faecalis) (6–8), and the early cell wall synthesis enzyme (MurA) (9). Ampicillin resistance has been achieved in an E. faecium strain devoid of Pbp5 (10) through the activity of a penicillin-/cephalosporin-insensitive l,d-transpeptidase and a d,d-carboxypeptidase where the phenotype is augmented by mutations that reduce the activity of StpA (11).
Eukaryote-like serine-threonine kinase/phosphatase systems are widespread in bacteria and have been implicated in many different cellular processes, including cell wall synthesis, cell division, and susceptibility to cell wall-active antibiotics (12). The effects of protein phosphorylation are numerous and include changes in regulation of transcription, protein activity, and protein-protein interactions.
The presence of class A Pbp PonA or PbpF was required for cephalosporin resistance in E. faecalis (4). Our own experiments performed in E. faecium yielded similar results (2), in which deletion of the E. faecium pbpF and ponA resulted in a strain that became susceptible to certain cephalosporins (ceftriaxone and cefepime, here referred to simply as cephalosporins) but not others (cefazolin and cefoxitin). These changes had no detectable impact on susceptibility to ampicillin. In addition, we found that phenotypic resistance to cephalosporins in these class A Pbp mutants could be induced by exposure to penicillin and could be readily selected in low-frequency mutants by growing large inocula on ceftriaxone-containing plates.
The present experiments were undertaken in an effort to understand the mechanisms of E. faecium cephalosporin susceptibility and resistance in the absence of class A Pbps. Our prior work indicated that cephalosporin resistance could be induced in class A Pbp mutants by exposure to penicillin, a phenotype that was reversible with removal of penicillin (2). To investigate the mechanisms of this phenotype, we examined changes in transcription among class A Pbp mutants after growth in the presence or absence of penicillin. We also observed that mutants stably resistant to cephalosporins could be selected from the class A Pbp-deficient strains on cephalosporin-containing media. We performed whole-genome sequencing in the generated cephalosporin-resistant mutants to identify changes linked to the resistant phenotype in the hopes that we might identify common pathways to cephalosporin resistance under the different circumstances. Finally, we used Pbp5 as bait in tandem affinity purification (TAP) experiments designed to identify proteins interacting with Pbp5 under conditions resulting in the cephalosporin-resistant phenotype (exposure to penicillin). We report the identification of a protein (Pbp5-associated protein [P5AP]) found in association with Pbp5 whose expression is increased in a cephalosporin-resistant mutant of the StpA-Stk eukaryote-like serine-threonine kinase/phosphatase system and by exposure to penicillin. In addition, overexpression of P5AP in a class A Pbp E. faecium mutant resulted in an increase in cephalosporin MIC, confirming its importance in phenotypic cephalosporin resistance in E. faecium.
RESULTS
Selection and characterization of cephalosporin-resistant mutants.
The strains and plasmids used in these experiments are listed in Table 1. E. faecium CV598 (ΔpbpF ΔponA ΔpbpZ Chlr) is a cephalosporin-susceptible mutant in which all three of the known class A Pbp genes have been deleted, with the chloramphenicol resistance (cat) gene used to replace pbpZ left in place (2). A 0.5 McFarland standard was made in brain heart infusion (BHI) from a fresh overnight plate, and 100 µl of each was inoculated onto BHI plates with a ceftriaxone disc. After 2 to 3 days, colonies appeared within the ceftriaxone zone and were recovered, purified for single colonies, and tested for susceptibility to ceftriaxone. One such ceftriaxone-resistant colony was chosen and sent for whole-genome sequencing. The mutations found in the resistant mutant (Cro1; ceftriaxone MIC, 250 µg/ml [Table 2]) are listed in Table S1 in the supplemental material. None of the mutations were in pbp5. The most interesting mutation found in Cro1 was in stk (ireK in E. faecalis), which has been identified as involved in the cephalosporin resistance phenotype of E. faecalis (6). The mutation introduced a premature stop codon 33 amino acids (aa) from the C terminus of the protein, in a region that extends beyond the PASTA domains typical of the extracellular portion of eukaryote-like serine-threonine kinases and is composed predominantly of serines (see Fig. S1 in the supplemental material). A search of the Swiss-Prot database yielded 34 E. faecium sequences, all with the serine tail. Similar serine-rich tails were present on stk homologues in Enterococcus durans, Enterococcus casseliflavus, Enterococcus mundtii, Enterococcus flavescens, and Peptoclostridium difficile Y384.
TABLE 1 .
Strains and plasmids used in this studya
| Strain or plasmid | Relevant phenotype | Resistance trait(s) | Reference or source |
|---|---|---|---|
| Enterococcus faecium strains | |||
| D344R | Wild type, penicillin and ceftriaxone resistant | Penr Cror Chls | 3 |
| CV598 | ΔpbpF ΔpbpZ ΔponA, ceftriaxone sensitive, derived from D344R, cat replacing pbpZ | Penr Cros Chlr Kans | 2 |
| CV558 | ΔpbpF ΔponA, ceftriaxone sensitive, derived from D344R; CAT replacing pbpF gene | Penr Cros Chlr Kans | 2 |
| CV571 | ΔpbpF ΔponA, ceftriaxone sensitive derived from CV558; CAT-deleted chromosomal insertion | Penr Cros Chls Kans | 2 |
| D344S | Spontaneous Δpbp5 from D344R | Pens Cros Chls Kans Rifr Fusr | 3 |
| Cro1 | Spontaneous ceftriaxone-resistant isolate from CV598 | Penr Cror Chlr Kans | This study |
| Cro2 | Spontaneous ceftriaxone-resistant isolate from CV598 | Penr Cror Chlr Kans | This study |
| LS002 | CV571 carrying pRIH011, with Pbp5 fused to TAP peptides | Penr Cros Chls Kanr | This study |
| LS030 | CV558 carrying pRIH39, with WT stpA-stk alleles under control of rhamnose promoter | Penr Cros Chlr Kanr | This study |
| LS031 | CV558 carrying pRIH36, with WT stpA and mutant stk under control of rhamnose promoter | Penr Cror Chlr Kanr | This study |
| LS017 | D344S carrying pRIH36, with WT stpA and mutant stk genes under control of rhamnose promoter | Pens Cros Chls Kanr | This study |
| LS106 | CRO2 carrying pRIH39, with WT stpA-stk alleles under control of rhamnose promoter | Penr Cros Chlr Kanr | This study |
| LS107 | CV571 carrying pRIH115, with pbp5 promoter US of TAP peptides | Penr Cros Chls Kanr | This study |
| LS108 | CV571 carrying pRIH113, with P5AP under control of rhamnose promoter | Penr Cros Chls Kanr | This study |
| Plasmids | |||
| pACYC184 | Low-copy-number cloning vector for growth in E. coli | Tetr Chlr | |
| pTCV-lac | Conjugative E. coli-E. faecium shuttle vector | Ermr Kanr | 20 |
| pBS1479 | C-terminal epitope tag consisting of a calmodulin binding peptide and a protein A moiety separated by a TEV cleavage site | Ampr | 22 |
| pCJK96 | E. coli-E. faecalis shuttle plasmid containing rhamnose-inducible promoter and regulator from E. faecalis | Ermr | 7 |
| pRIH11 | pbp5-TAP tag fusion in the SmaI site of pTCV-lac | Ermr Kanr | This study |
| pRIH39 | Rhamnose promoter and regulator from E. faecalis and WT stpA-stk alleles in SmaI site of pTCV-lac | Ermr Kanr | This study |
| pRIH36 | Rhamnose promoter and regulator from E. faecalis and Cro1 stpA-stk alleles in SmaI site of pTCV-lac | Ermr Kanr | This study |
| pRIH115 | pbp5 promoter and TAP tag in SmaI site of shuttle vector pTCV-lac | Ermr Kanr | This study |
| pRIH113 | Rhamnose promoter and regulator from pCJK96 US of P5AP coding sequence from D344R, in SalI sites of pTCV-lac | Kanr | This study |
Abbreviations: CAT, chloramphenicol acetyltransferase; WT, wild type; US, upstream; Amp, ampicillin; Chl, chloramphenicol; Cro, ceftriaxone; Erm, erythromycin; Fus, fusidic acid; Kan, kanamycin; Pen, penicillin; Rif, rifampin; Tet, tetracycline.
TABLE 2 .
MICs of different antibiotics against cephalosporin-susceptible E. faecium CV598 (ΔpbpF ΔponA ΔpbpZ) and two cephalosporin-resistant mutants, Cro1 and Cro2, and E. faecium CV558 (ΔponA ΔpbpF) alone or carrying cloned versions of stpA-stk from D344R or from the Cro1 mutant in which the terminal portion of Stk has been deleteda
| Strain (genotype) | Plasmid | Growth condition | MIC (µg/ml) of drug: |
||||
|---|---|---|---|---|---|---|---|
| PenG | Amp | Cro | Van | Spec | |||
| CV598 (ΔpbpF ΔponA ΔpbpZ) | None | Uninduced | 50 | 25 | 1.56 | ND | ND |
| Cro1 (ΔpbpF ΔponA ΔpbpZ stk mutant) | None | Uninduced | 50 | 25 | 250 | ND | ND |
| Cro2 (ΔpbpF ΔponA ΔpbpZ stpA mutant) | None | Uninduced | 50 | 25 | >1,000 | ND | ND |
| CV558 (ΔpbpF ΔponA) | None | Uninduced | 100 | 50 | 3.13 | 0.78 | 31.25 |
| LS030 (ΔpbpF ΔponA) | pRIH39 (stpA-stk WT) | Uninduced | 50 | 50 | 6.25 | 0.2 | 31.25 |
| LS030 (ΔpbpF ΔponA) | pRIH39 (stpA-stk WT) | Induced | 25 | 50 | 3.13 | 0.2 | 31.25 |
| LS031 (ΔpbpF ΔponA) | pRIH36 (stpA-stk Cro1) | Uninduced | 100 | >100 | >100b | 1.56 | 31.25 |
| LS031 (ΔpbpF ΔponA) | pRIH36 (stpA-stk Cro1) | Induced | 100 | 100 | >100 | 0.78 | 15.63 |
Abbreviations: WT, wild type; PenG, penicillin; Amp, ampicillin; Cro, ceftriaxone; Van, vancomycin; Spec, spectinomycin; ND, not done.
Promoter does not completely suppress transcription.
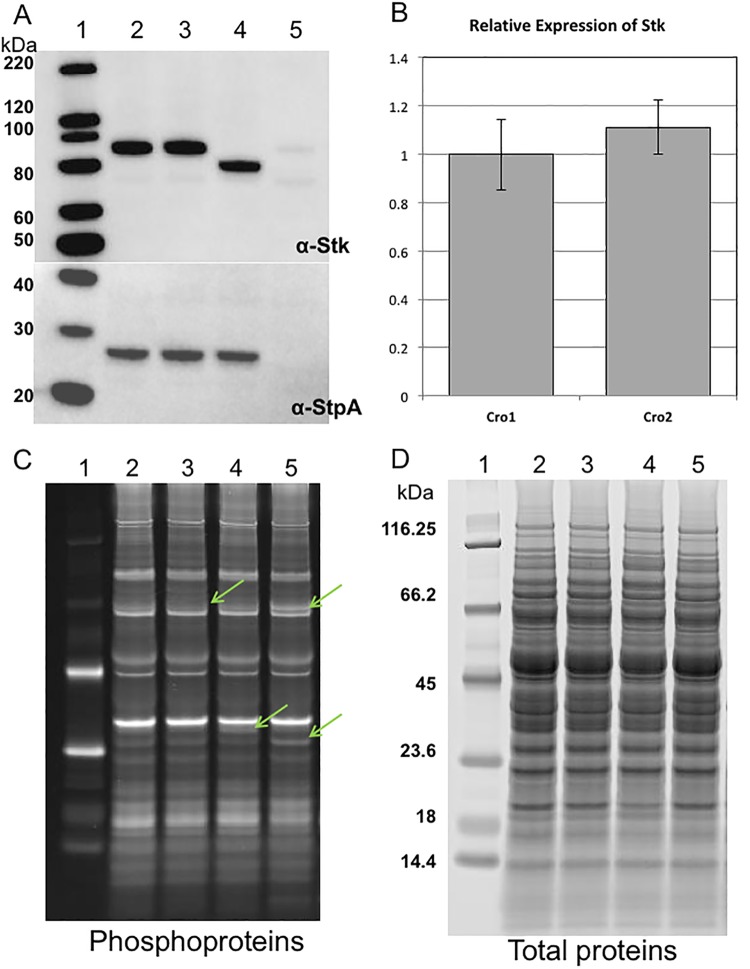
E. faecium CV558 (ΔpbpF ΔponA Cmr) and CV571 (ΔpbpF ΔponA) are double class A Pbp mutants with the same cephalosporin-susceptible phenotype as CV598. They grow more robustly than CV598 and are more amenable to genetic manipulation and so were used for most cloning and complementation experiments. We attempted to delete stk from CV571 without success. stk exists in an operon downstream of and cotranscribed with its cognate phosphatase (stpA). Prior work in E. faecalis suggested that cloning the two genes together yields better results than either alone (C. Kristich, personal communication). We amplified and cloned both the native and mutant stpA-stk coding sequences into the shuttle plasmid pTCV-lac under the control of a rhamnose-inducible promoter and reintroduced both into E. faecium CV558. There was no change in ceftriaxone MIC associated with introduction of the native coding sequence into CV558. However, introduction of the mutant stk with the native stpA was associated with rhamnose-inducible resistance to ceftriaxone (Table 2), confirming the association of this mutation with the cephalosporin-resistant phenotype. This indicates that the mutated gene is dominant over the wild type and suggests that the mutation results in an augmentation of kinase activity. Western blot analysis of crude cellular extracts from CV598 and the Cro1 mutant with anti-Stk antibody confirmed that a protein of smaller size was produced in the Cro1 mutant (Fig. 1A).
FIG 1 .
Expression of Stk and StpA in CV598, CV598 plus penicillin (10 µg/ml), Cro1 mutant, and Cro2 mutant from whole-cell lysates at mid-exponential growth phase. (A) (Top) Western blot analysis of CV598 (lane 2), CV598 plus penicillin (lane 3), Cro1 mutant (lane 4), and Cro2 mutant (lane 5) using polyclonal antibody to Stk. The smaller size of the band in lane 4 confirms the truncation of the terminus of Stk. Only a very faint Stk band appears for the Cro2 mutant, suggesting that translation of the full StpA and that of Stk are linked, possibly through a translational coupling mechanism. (Bottom) Western blot analysis of whole-cell lysates from strains listed in the upper panel using a polyclonal antibody to StpA. As expected, there is no band in the expected size range of StpA for the Cro2 mutant, whose predicted amino acid sequence is truncated approximately halfway through the protein. (B) Relative expression of Stk in the Cro1 and Cro2 mutants. Expression levels of 16S rRNA and tufA were used as references. Error bars indicate the standard error of the mean for biological duplicates. Results are representative of two independent experiments. (C) Phosphoprotein analysis, by phosphoprotein gel-stained SDS-PAGE of whole-cell lysates of strains depicted in panel A. Arrows indicate minor differences in the strains. (D) Total protein analysis of whole-cell lysates of strains depicted in panel A, revealing equivalent amounts of total protein in the preparations.
In an effort to determine whether mutations in stpA-stk represent a common pathway to cephalosporin resistance in our class A Pbp mutants, we selected another round of ceftriaxone-resistant mutants of CV598. Two of these were purified and passaged as before, and stpA and stk were amplified and sequenced. In one of these two mutants (Cro2; ceftriaxone MIC, 1,000 µg/ml [Table 2]), we detected a T-to-A change at position 369 of stpA, which converts a tyrosine to a stop codon after 123 amino acids of a protein predicted to be 246 aa in length, resulting in a truncated and presumably nonfunctional protein. Transformation of the Cro2 mutant with the native stpA-stk resulted in a return of cephalosporin susceptibility (see Fig. S2 in the supplemental material), suggesting that the mutation resulted in a nonfunctional protein and that the phosphatase activity is associated with cephalosporin susceptibility in these strains. Western blot analysis of crude cellular extracts from the Cro2 mutant (Fig. 1A) did not identify StpA, which, given the Cro2 mutation, would be expected to be half the size of the full protein and potentially unstable. Surprisingly, only a very faint band was observed corresponding to the expected location for the Stk band, despite quantities of message comparable to those found in the Cro2 mutant by reverse transcription-quantitative PCR (RT-qPCR) (Fig. 1B), suggesting that translation of the kinase may be dependent upon full translation of the phosphatase. A comparison of phosphorylated proteins by phosphoprotein gel staining of SDS-PAGE-separated proteins from crude cellular extracts of the Cro1 and Cro2 mutants and CV598 with or without penicillin exposure showed minor differences in both the degree of phosphorylation and the appearance of newly phosphorylated proteins between the strains (Fig. 1C). SYPRO Ruby was used as a poststain to confirm equivalent amounts of total protein loaded and gave a snapshot of the relative phosphorylation state differences compared to the phosphorylated protein profiles (Fig. 1D).
Proteins associated with Pbp5.
Based on the assumption that an unidentified glycosyltransferase might be physically associated with Pbp5 during the process of peptidoglycan synthesis in the double and triple class A Pbp mutants, we analyzed proteins associated with Pbp5 under conditions that confer resistance to cephalosporins. We used the tandem affinity purification (TAP) technique (13) with Pbp5 as the bait protein to isolate, and mass spectrometry to identify, Pbp5-associated proteins after growth of CV571 either with or without exposure to penicillin (10 µg/ml). Proteins associated with the TAP tag without Pbp5 were also analyzed to assess background contaminants. A list of the associated proteins is shown in Table S2A in the supplemental material. As expected, peptides derived from Pbp5 were most frequently identified. Also identified in association with Pbp5 and at a higher frequency when cells were incubated with penicillin was a protein of unknown function that we have designated P5AP (Pbp5-associated protein) (EDAG_01115 [protein identifier {ID} EFD09967 from the genome of E. faecium D344SRF]) referred to in the mass spectrometry data set as H8LA73_H8LA73_ENTFU from the E. faecium strain Aus0004 closed genome used as a reference. Peptides from this protein predominated in penicillin-treated cells, were identified with considerably lower frequency in the absence of penicillin, and were not identified at all in the purified protein complexes from the TAP tag without Pbp5 in the absence of penicillin (see Table S2B). The conditions under which P5AP is found with greater frequency are the conditions under which greater levels of cephalosporin resistance are achieved.
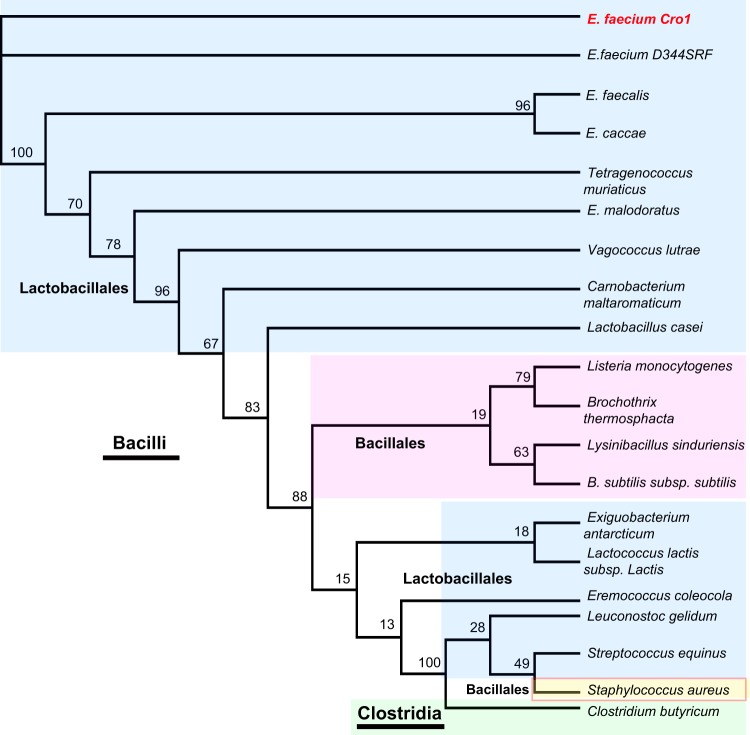
All 244 available E. faecium genome sequences in the EnsembleBacteria database contained P5AP, suggesting that it is part of the E. faecium core genome. The predicted P5AP protein is 522 amino acids in length and contains the domains of unknown function DUF4097 (IPR025164) and DUF4098 (IPR025386); these domains are conserved in all identified homologues of P5AP in different Enterococcus species. Additionally, we identified proteins with both DUF4097 and DUF4098 domains in other Lactobacillales, including Lactobacillus, Aerococcus, and Carnobacterium. Interestingly, the sequences retrieved from Leuconostoc, Streptococcus, and Lactococcus are highly divergent (Fig. 2; see also Fig. S3 and Table S3 in the supplemental material) and do not cluster with other Lactobacillales. In more distantly related Firmicutes, we identified DUF4097-DUF4098-containing proteins in the Bacillales, including Bacillus, Listeria, and Staphylococcus and in the more distant Clostridia.
FIG 2 .
Maximum likelihood tree. P5AP homologues in Firmicutes. E. faecium Cro1 and E. faecium D344SRF are identical. P5AP from E. faecium is closer to other enterococcal P5AP sequences; interestingly, the closely related Streptococcus, Lactococcus, and Leuconostoc are highly divergent from the Enterococcaceae. The Bacillales all form a single branch with the exception of Staphylococcus. E. faecium Cro1 is highlighted in red. The Lactobacillales are shown in light blue. The Bacillales are shown in light magenta. Staphylococcus is shown in light yellow. Clostridium is shown in green.
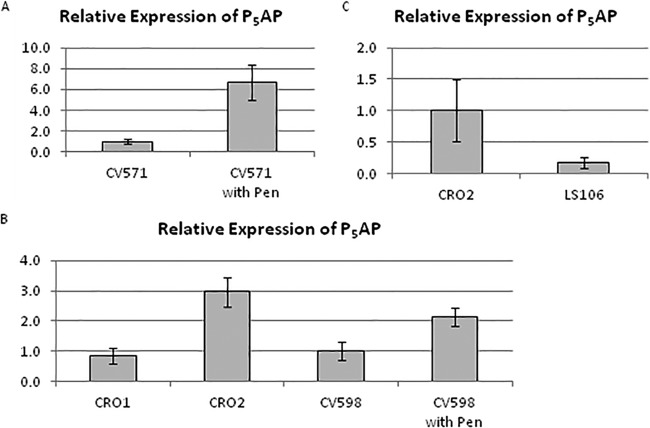
Microarray experiments indicated that the P5AP coding sequence was expressed at higher levels in CV571 after penicillin exposure (see Table S4 in the supplemental material), a result that was confirmed by RT-PCR (Fig. 3A). Expression of the P5AP coding sequence was not significantly increased in the Cro1 mutant but was increased 2- to 10-fold in the Cro2 mutant (Fig. 3B). Expression was also increased 2-fold in CV598 after penicillin exposure (Fig. 3B), as well as in clinical isolates C68 (4.8-fold) and D344R (2.8-fold) after exposure to penicillin. Expression of P5AP was not increased in the Pbp5− strain D344SRF (data not shown), suggesting that interaction of penicillin with Pbp5 may be the event leading to the increased expression. Transformation of Cro2 with a plasmid expressing wild-type stpA-stk resulted in a substantial reduction in P5AP gene expression (Fig. 3C). Transformation of CV571 by the P5AP coding sequence cloned under the control of a rhamnose-inducible promoter revealed a rhamnose-induced decrease in susceptibility to ceftriaxone (Table 3). Interestingly, rhamnose induction was also associated with a 16-fold reduction in susceptibility to ampicillin.
FIG 3 .
Expression of the P5AP gene is induced in response to exposure to penicillin or following acquisition of a nonsense mutation in stpA. Cultures were grown in BHI broth to mid-log phase, and penicillin was added at 12.5 µg/ml (one-half of the MIC). After 30 min, cells were harvested and frozen for RNA preparation. Expression levels of 16S rRNA and tufA were used as references. Error bars indicate the standard error of the mean for biological duplicates. Results are representative of two independent experiments. (A) Relative gene expression of P5AP in ceftriaxone-susceptible double class A Pbp mutant CV571 in response to growth in penicillin. (B) Relative gene expression of P5AP in the Cro1 and Cro2 mutants and of ceftriaxone-susceptible triple class A Pbp mutant CV598 in response to growth in penicillin. (C) Relative gene expression of P5AP in the Cro2 mutant alone and transformed with the wild-type stpA-stk.
TABLE 3 .
MICs of P5AP clones in CV571
| Strain (genotype) | Plasmid | Growth condition | MIC (µg/ml) of druga: |
|||
|---|---|---|---|---|---|---|
| PenG | Amp | Cro | Chlor | |||
| CV571 (ΔpbpF ΔponA) | None | Uninduced | 25 | 12.5 | 1.56 | 3.13 |
| CV571 (ΔpbpF ΔponA) | None | Induced | 25 | 6.25 | 1.56 | 6.25 |
| LS108E (ΔpbpF ΔponA) | pRIH113 (P5AP gene) | Uninduced | 50 | 50 | 6.25 | 3.13 |
| LS108E (ΔpbpF ΔponA) | pRIH113 (P5AP gene) | Induced | 50 | 100 | 25 | 3.13 |
Abbreviations: PenG, penicillin G; Amp, ampicillin; Cro, ceftriaxone; Chlor, chloramphenicol.
DISCUSSION
Eukaryote-like serine-threonine kinases are ubiquitous in Gram-positive bacteria and have been shown to be involved in a variety of cellular functions, including virulence, colonization ability, cell envelope biosynthesis, and antimicrobial resistance, among others (12). Our findings that stpA and stk are involved in cephalosporin resistance in E. faecium are expected given the results of prior studies but offer further insight into the function of these genes in this species. Kristich and colleagues (6, 7) have established that the orthologue of Stk in E. faecalis (IreK) is required for resistance to cephalosporins and is also involved in colonizing ability in a mouse gastrointestinal model. They also established that IreK is a self-phosphorylating kinase and that IreP works as a phosphatase to modulate the activity of IreK in promoting resistance to cephalosporins, at least in part by serine and threonine dephosphorylation in the IreK active site. Under the conditions of their assay, they found no evidence that IreK phosphorylated IreP. They have identified a single additional target for IreK phosphorylation, designated IreB (14), but there is as yet no defined function for this protein.
A previous study (8) found an impairment of function by a mutation in stpA to be associated with modestly increased levels of resistance to ampicillin in E. faecium D344S, a strain that is cephalosporin and ampicillin susceptible through spontaneous deletion of 160 kb of genomic DNA that included pbp5 (15). High-level resistance to ampicillin was achieved in this strain through a series of mutations leading to predominant synthesis of peptidoglycan by an l,d-transpeptidase whose tetrapeptide substrate was created by the activity of a d,d-carboxypeptidase that is variably present in different E. faecium strains. Mutations leading to decreased function of the StpA or increased expression of the d,d-carboxypeptidase alone resulted in only modest increases in ampicillin resistance, but the combination of the two mutations led to very high levels of resistance. Their studies also established that StpA, similar to IreP, is capable of dephosphorylating its cognate kinase Stk.
Our data indicate that, as in E. faecalis, cephalosporin resistance in E. faecium expressing low-affinity Pbp5 is reciprocally controlled by the phosphatase and kinase activities of StpA and Stk. Eukaryote-like serine-threonine kinases have a characteristic structure in which the kinase domain is cytoplasmic and connected to an external series of PASTA (PBP- and serine/threonine kinase-associated) domains thought to interact with nascent peptidoglycan in a manner that detects modifications potentially deleterious to the bacterium (12). The structure of E. faecium Stk differs from most others in that the PASTA domains are connected to a “tail” that is composed predominantly of serines. The mutation observed in Stk in Cro1 occurred in this tail end of the molecule, truncating the serine string by 33 amino acids, as evidenced by the Western blot showing a smaller protein in the mutant strain (Fig. 1A). We presume that this mutation leads to an increase in Stk activity, since cloning and expressing the stpA-stk coding sequence with the truncating mutation in a strain also expressing the native Stk confers cephalosporin resistance on our cephalosporin-susceptible class A Pbp mutants. Given that the truncation occurs in the extracellular portion of the molecule, we propose that it may result in a constitutive activation of the cytoplasmic kinase portion, with the resultant increase in the resistance to cephalosporins. In a similar fashion, expression of the wild-type stpA-stk in Cro2, which has a mutation in stpA predicted to truncate the protein to half its normal length, presumably eliminating its activity, was associated with the return of cephalosporin susceptibility, suggesting that the Cro2 mutation results in loss of function of StpA and cephalosporin resistance.
Similar to observations by Kristich and colleagues for E. faecalis (6), we observed a considerably higher level of cephalosporin resistance associated with the loss of StpA activity than with the presumably increased activity of Stk. They observed that a strain in which ireP-ireK was deleted exhibited increased susceptibility compared to the wild type and that a strain with an IreK with three active site threonines replaced by a phosphomimetic uncleavable by IreP exhibited high-level cephalosporin resistance even in the presence of active IreP, concluding that phosphorylation of IreK was essential for the expression of cephalosporin resistance. In contrast, Western blot analysis of our Cro2 mutant using the Stk and the StpA antibodies detected a faint band corresponding to the size of the wild-type Stk and failed to demonstrate the presence of StpA (Fig. 1A). RT-PCR analysis of stpA-stk revealed the expected quantity of transcript (Fig. 1B), suggesting that translation of the stk message, which lies downstream of stpA, may depend upon translation of the terminal portion of stpA. The fact that the Cro2 mutant shows high levels of resistance to cephalosporins despite barely detectable levels of Stk on Western blot analysis suggests that the absence of StpA is the predominant factor in determining the level of cephalosporin susceptibility in E. faecium. As StpA has been shown to dephosphorylate Stk (8), the complete absence of StpA may result in a higher overall quantity of phosphorylated Stk, despite a considerably lower absolute quantity of protein (phosphorylated and unphosphorylated). It is also possible that, in contrast to E. faecalis, the primary role of Stk in the cephalosporin-resistant phenotype of class A Pbp-deficient E. faecium is through phosphorylation of StpA, which according to this model would serve to moderate the StpA phosphatase activity and thereby augment the resistance to cephalosporins. This model is at variance with the findings in E. faecalis (6), where in vitro experiments failed to demonstrate IreK phosphorylation of IreP. Alternatively, StpA may dephosphorylate proteins other than Stk that are involved in cephalosporin resistance. It is clear that StpA plays an important role in expression of P5AP, since introduction of wild-type stpA into the Cro2 mutant reduces P5AP gene transcription and restores cephalosporin susceptibility. We are in the process of planning experiments to define the links between StpA activity and P5AP gene transcription.
When we originally decided to delete the class A Pbps of E. faecium and test the impact of those deletions on β-lactam resistance, our assumption was that Pbp5 could likely work with the previously described class A glycosyltransferases and that, lacking those glycosyltransferase partners, it would not be able to participate in cell wall synthesis. Since it has been established that Pbp5 is the sole Pbp that confers β-lactam resistance in E. faecium (strains lacking Pbp5 are fully susceptible to β-lactams) (3, 16), we anticipated that elimination of partner glycosyltransferases would yield susceptibility to a wide range of β-lactams. To our surprise, deletion of ponA and pbpF yielded strains that were susceptible to only a narrow range of cephalosporins (ceftriaxone and cefepime, which share a side chain) and retained resistance to other β-lactams. This susceptibility was reversible by exposure to penicillin (to which the strains are resistant), and resistant mutants were readily selected on cephalosporin-containing agar. In short, the phenotype was not consistent with a model in which Pbp5 was unable to participate in peptidoglycan synthesis.
It has recently been reported (17) that the activity of ceftaroline against methicillin-resistant Staphylococcus aureus involves binding of ceftaroline to an allosteric site on low-affinity class B Pbp2a, triggering a conformation change that opens up the active site, allowing binding of a second ceftaroline molecule and inhibition of Pbp2a activity. These investigators reported that a similar allosteric site appeared to be present in E. faecium Pbp5. With this model in mind, we hypothesize that E. faecium Pbp5 has an allosteric site, capable of binding ceftriaxone and cefepime and creating a conformational change in the active site that allows binding and inhibition by a second cephalosporin molecule. We propose that the interaction between Pbp5 and other proteins, either class A Pbps (PonA or PbpF), P5AP, or a combination of these proteins, either precludes cephalosporin binding to the allosteric site or prevents the subsequent conformational change that normally occurs after allosteric binding. Deleting ponA and pbpF results in the interaction between cephalosporins and the allosteric site and hence susceptibility. Exposure to penicillin in these cephalosporin-susceptible strains results in increased expression of P5AP, which, we hypothesize, interacts with Pbp5 in a manner that precludes either allosteric binding or the resulting structural changes. Constitutive acquisition of cephalosporin resistance can occur in this setting through a null mutation in stpA, presumably through increased phosphorylation of a transcription factor for the P5AP gene.
At this time, we have no compelling data to suggest a specific activity for P5AP. It shares little similarity with proteins of known function. Our sequence searches retrieved homologous sequences in Enterococcus species, other Enterococcaceae, other Lactobacillales (Lactobacillus, Streptococcus, Lactococcus), Bacillales (Staphylococcus and Listeria), and Clostridia (Clostridium). Our search found that proteins containing DUF4097 (IPR025164) and DUF4098 (IPR025386) are not particularly conserved. The DUF4097/DUF4098-containing proteins in Streptococcus and Lactococcus are highly divergent from those in the enterococci. It is interesting that Enterococcus and Listeria are two species that are intrinsically resistant to cephalosporins, but their P5AP sequences share only 22.6% identity. While it is tempting to speculate that P5AP serves as a glycosyltransferase, there are no suggestions in the structure that it has such activity. Using the secondary structure prediction program PSIPRED, it was observed that residues 1 to 235 are mostly alpha helical while beta-sheets predominate in the C-terminal domain (residues 240 to 520). There are no obvious transmembrane regions or export signals, though its close association with Pbp5 strongly suggests that it acts in the extracellular milieu, since Pbp5 is predominantly an extracellular protein. Experiments are planned to better define the structure of P5AP and its interaction with Pbp5 to further refine our model for its involvement in E. faecium resistance to cephalosporins.
MATERIALS AND METHODS
Bacterial strains, growth media, chemicals, and microbiological techniques.
The strains used in this study are listed in Table 1. E. faecium cultures were grown in either brain heart infusion (BHI) broth (Fluka) or MM9YE minimal medium containing either glucose (2%) or ribose (0.2%) and rhamnose (2%) (1) at 37°C. Escherichia coli was grown in Luria-Bertani broth (LB) at 37°C. Concentrations of antibiotics for selection were as follows: kanamycin, 50 µg/ml (E. coli) or 1.5 µg/ml (E. faecium); chloramphenicol, 25 µg/ml (E. coli) or 10 µg/ml (E. faecium); rifampin, 100 µg/ml; fusidic acid, 50 µg/ml. All antibiotics and chemicals were obtained from Sigma-Aldrich unless otherwise stated. Disc diffusion assays were carried out by standard methods on BHI agar. Zone sizes were determined by visual inspection after 18 to 24 h of incubation. Microbroth MIC determinations were carried out by standard methods except that cells were grown in either BHI or MM9YE with either glucose (noninducing medium) or rhamnose (inducing medium) (1). For phosphoproteins and Western blot assays, E. faecium cultures were grown in BHI broth until mid-logarithmic stage and then divided and treated with or without 10 µg/ml penicillin G (Sigma catalog no. P7794) for 1 h at 37°C with shaking at 200 rpm. Cells were harvested from 50-ml culture volumes, and cell pellets were stored at −80°C. For the TAP method, cells were grown in BHI with or without 10 µg/ml penicillin until mid-log stage. Cells were washed in phosphate-buffered saline, and pellets were stored at −80°C.
Isolation of E. faecium ceftriaxone-resistant clones.
The parental strains CV558 and CV598 were streaked on BHI agar from frozen stock and grown overnight. McFarland standards of 0.5 were made in BHI broth and spread onto BHI plates. A single ceftriaxone disc (30 µg) was applied to each plate, which was incubated for 48 h at 37°C. Zones of inhibition around the antibiotic discs were observed, with resistant colonies growing in the zone. Multiple colonies from the ceftriaxone zones were selected and streaked for single colonies on BHI agar. A single colony from each streak was selected and restreaked for a total of 10 passages. Isolates were then retested by disc diffusion, and a resistant isolate was selected for whole-genome sequencing. A second mutant was isolated as before and screened by sequencing the stpA and stk genes.
Genome sequencing and analysis to identify mutations in ceftriaxone-resistant strains.
Genomic DNA was extracted from overnight cultures. The genomic DNA libraries were constructed using a previously described method (18) with minor modifications. Genomic libraries were pooled for sequence analysis on one lane of an Illumina HiSeq 2000 ll sequencing system. Assembly was accomplished by alignment with the E. faecium Aus0004 genome (19). Genomics comparisons between the sequenced strains were used to identify insertions, deletions, and single point mutations that may be responsible for the observed resistance profiles. High-confidence mutations in coding sequences that were identified initially were confirmed by Sanger sequencing of PCR products.
Molecular biology techniques.
DNA-modifying enzymes and kits were purchased from New England Biolabs, unless otherwise noted. Primers were obtained from Sigma. All PCRs were carried out using Phusion high-fidelity polymerase from New England Biolabs, according to the manufacturer’s directions. Plasmid preparations were carried out using Qiagen midiprep kits. Enterococcal genomic DNA was made using the Qiagen DNeasy kit.
Construction of plasmids and transfer to E. faecium.
Due to the homology seen to ireK identified in E. faecalis (1), stk from Cro1 was chosen for further analysis. Mutant and wild-type versions of the stk gene, along with the upstream stpA gene, were cloned by PCR into the E. coli/Gram-positive shuttle vector pTCV-lac (20) behind the rhamnose-inducible promoter from pCJK96 by standard methods. This plasmid was transformed into E. coli SM10 and mated into E. faecium in BHI broth. Transconjugants were selected with kanamycin and either chloramphenicol (for CV558 and CRO2) or rifampin plus fusidic acid (for D344S).
The tandem affinity purification (TAP)-pbp5 fusion was constructed by cloning a PCR product of Pbp5 coding sequence plus ~800 bp upstream into pBS1479 (13). After sequence confirmation, the fusion protein was transferred into pTCV-lac. This plasmid was electroporated into CV571, selecting with kanamycin. For the TAP construct with the Tap tag alone, the Pbp5 promoter region (~800 bp) was independently cloned into pBS1479. The SapI/SmaI fragment was transferred into pTCV-lac as before.
The P5AP overexpression plasmid was constructed by cloning the coding sequence into SmaI/SalI sites of pTCV-lac (eliminating lacZ and ermB) behind the rhamnose-inducible promoter from pCJK96. This plasmid was electroporated into CV571.
Gene expression studies.
Relevant strains were grown with shaking at 37°C to an A600 of between 0.4 and 0.5. At that point, aliquots had penicillin added at a final concentration of half the MIC value and were grown for a further half hour with shaking. Cells were broken open with glass beads (Lysing Matrix B; MP Biomedical) using a mini-BeadBeater (BioSpec), and RNA was purified using a Qiagen RNeasy minikit. Quantitative reverse transcriptase PCR was carried out using a Bio-Rad iTaq universal SYBR one-step kit and a CFX98 real-time PCR cycler. Relative gene expression was calculated using the Pfaffl method (24) and normalized relative to expression of 16S rRNA and ith mRNA.
Microarray analyses.
In triplicate, total bacterial RNA was isolated from E. faecium strain CV571 following a 30-min treatment with 10 µg/ml penicillin or no drug. A total of 10 µg RNA from each sample was reverse transcribed using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). The resulting cDNA was purified using QIAquick PCR purification kits (Qiagen, Germantown, MD), fragmented with DNase I (Ambion, Carlsbad, CA), and then 3′ biotinylated using Bioarray terminal labeling kits (Enzo Life Sciences, Farmingdale, NY). A total of 1.5 µg of labeled material from each sample was hybridized to custom-made E. faecium Affymetrix GeneChips (part number PMDefcma520788F) that contained a total of 4,395 probe sequences representing 3,898 open reading frames and 497 intergenic E. faecium annotated GenBank entries at the time of array design. GeneChips were then processed according to the manufacturer’s recommendations for prokaryotic arrays (Affymetrix, Santa Clara, CA). Differentially expressed transcripts were identified as RNA species that demonstrated a 1.5-fold increase or decrease in expression in penicillin-challenged CV571 cells in comparison to mock treatment (t test, P = 0.05).
Preparation of protein lysates for SDS-PAGE and phosphoprotein analysis.
All chemicals were obtained from Sigma unless otherwise specified. Cell pellets were washed twice in 10 mM Tris-HCl, pH 7.5, 50 mM NaCl buffer, and resuspended at 50 mg/ml in lysis buffer containing 10 mM Tris-HCl, pH 7.5, 50 mM NaCl with 5 mM EDTA, 1× HALT protease inhibitor cocktail, and 0.5× HALT phosphatase inhibitor cocktail (Thermo Scientific). Cells were processed 4 times for 30 s each in Lysing Matrix B tubes using the mini-BeadBeater (BioSpec Products), lysates were clarified, and protein concentrations were determined using the 2-D Quant kit (GE Healthcare). The lysates were precipitated according to the Invitrogen Pro-Q Diamond phosphoprotein gel stain (Molecular Probes) recommendations using the methanol-chloroform protocol. The PeppermintStick phosphoprotein molecular weight standards (Molecular Probes) were diluted and treated as recommended and used for both molecular weight markers and positive and negative controls for the phosphoprotein gel stain. Protein samples solubilized in 1× LDS sample buffer (Invitrogen) were separated using Invitrogen NuPAGE 4 to 12% Bis-Tris 1-mm gels with MES SDS (2-morpholinoethanesulfonic acid sodium dodecyl sulfate) running buffer and NuPAGE antioxidant for 40 min at 200 V.
The Invitrogen Pro-Q Diamond protocol for in-gel staining of phosphoproteins was followed except for a modification using 3-fold-diluted Pro-Q Diamond phosphoprotein stain, and the incubation step increased to 120 minutes per the work of Agrawal and Thelen (21). The gel was imaged using the Bio-Rad ChemiDoc XRS Imager with the trans-UV setting at 302-nm excitation and the standard emission filter with a range of 535 to 640. The gel was further analyzed by staining with SYPRO Ruby protein gel stain using the Rapid Protocol method as described in the SYPRO Ruby protocol (Molecular Probes). Duplicate 4 to 12% Bis-Tris NuPAGE gels were run for Western blot assays using MagicMark XP Western protein standard (Invitrogen) for molecular weight estimation.
Western blot analysis.
After electrophoresis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes using the iBlot (Invitrogen) dry blotting system. Polyclonal rabbit anti-StK and anti-StpA antibodies were used at recommended dilutions of 1/2,000 and 1/1,000, respectively (8). A Bis-Tris detergent-buffered saline solution with Hammersten casein was used for the block and antibody dilutions (Invitrogen; Western Breeze blocker/diluent). Goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (Invitrogen) was used at a 1/10,000 dilution, and the proteins were detected with Clarity Western enhanced chemiluminescence (ECL) blotting substrate (Bio-Rad).
Tandem affinity purification.
The C terminus of the pbp5 gene in strain CV571 was epitope tagged with the TAP tag, consisting of a calmodulin binding peptide and a protein A moiety separated by a tobacco etch virus (TEV) cleavage site, allowing for a two-step affinity purification of protein complexes with minimal background contaminants. E. faecium strains LS002 and LS107, carrying the Pbp5 fusion with the TAP tag and the TAP tag alone, respectively, were used for the TAPs. Cell pellets were resuspended in lysis buffer containing 10 mM Tris-HCl (pH 8), 100 mM NaCl, 0.1% NP-40, and HALT protease inhibitors (Thermo Scientific) and disrupted by bead beating in a BioSpec BeadBeater, and the lysate was clarified by centrifugation. The TAP was performed according to the protocol of Puig et al. (22) with slight modifications. IgG-Sepharose binding was performed in a 50-ml conical tube with 600 μl of IgG Sepharose 6 Fast Flow bead suspension, and the tube was incubated overnight at 4°C. The unbound fraction was removed by centrifugation, and the resin was resuspended in 10 ml of IgG-Sepharose binding buffer and transferred into an 0.8- by 4-cm Poly-Prep column (Bio-Rad). The resin was subsequently washed with binding buffer followed by TEV cleavage buffer (10 mM Tris-HCl, pH 8, 150 mM NaCl, 0.1% NP-40, 0.5 mM EDTA, and 1 mM dithiothreitol). Cleavage was performed in the column with 300 units of ProTEV Plus (Promega) in TEV cleavage buffer rotating overnight at 30°C. The eluate was collected by gravity flow and treated according to the protocol in the work of Puig et al. (22). Calmodulin affinity purification of the TEV-cleaved material was performed at 4°C for 2 h with rotation in a second column using 600 μl of calmodulin affinity resin suspension (Agilent Technologies). Unbound material was removed by gravity flow followed by two successive washes with calmodulin binding buffer (10 mM Tris-HCl, pH 8, 150 mM NaCl, 1 mM magnesium acetate, 1 mM imidazole) containing first 0.1% NP-40 and then 0.02% NP-40. The protein complexes were eluted with calmodulin elution buffer containing 10 mM Tris-HCl (pH 8), 150 mM NaCl, 1 mM magnesium acetate, 1 mM imidazole, 2 mM EGTA, and 10 mM beta-mercaptoethanol after a 5-min incubation at 4°C.
The eluted protein complexes were precipitated with trichloroacetic acid (TCA) according to the TCA-acetone protocol obtained from the Taplin Mass Spectrometry Facility and submitted for mass spectrometric processing and analysis.
Phylogenetic analysis of P5AP.
To identify P5AP homologues, we used the HMMER online server (http://www.ebi.ac.uk/Tools/hmmer/search/phmmer) to search for proteins with the DUF4097 and DUF4098 domains. We used default values and used the UniProtKB database. The HMMER search against UniProtKB retrieved 795 proteins, 781 from Bacteria and 705 of those from Firmicutes, mostly concentrated in the Bacilli (558 proteins; see Table S3 in the supplemental material). We selected representative sequences from Enterococcus, other Lactobacillales, Bacillales, and two Clostridiales for phylogenetic comparison. For phylogenetic analyses, protein sequences were aligned using Clustal Omega. Phylogenetic analyses were then carried out using PhyLM online (23) using the LG substitution model, NNI tree searching, and aLRT SH-Like branch support. The resulting tree and matrix were visualized with Geneious 8.1.5.
Microarray data accession number.
The GeneChip format and corresponding data files were deposited in the NCBI Gene Expression Omnibus repository under accession number GSE77436.
SUPPLEMENTAL MATERIAL
Stk protein sequences from CV598 and Cro1. Download
Extracellular domain of the eukaryote-like serine-threonine kinase from CV598, Cro1, and E. faecalis OG1RF. Download
Ceftriaxone disc assay of the Cro2 mutant. (Left) Cro2 mutant growing all the way up to the disc. (Right) Large zone around ceftriaxone disc in Cro2 mutant transformed with wild-type stpA-stk. Download
Extended maximum likelihood tree. P5AP homologues from Firmicutes. E. faecium Cro1 is highlighted in red. P5AP from E. faecium is closer to other enterococcal P5AP sequences (blue branches); the closely related Streptococcus, Leuconostoc, and Lactococcus are highly divergent from the Enterococcaceae, making the Lactobacillales lie in three separate clusters (light blue boxes). The Bacillales form all a single branch (light magenta box) with the exception of Staphylococcus (shown in a yellow box with an asterisk). The Clostridia (Clostridium butyricum and Clostridium pasteurianum) are shown in light green. Download
High-stringency mutations identified in 558CRO and 598CRO1 by next-generation sequencing.
Mass spectrometry peptide and protein data for TAP-purified protein complexes.
Distance matrices for phylogenetic analysis.
Gene expression data for CV571 with and without penicillin exposure.
ACKNOWLEDGMENTS
Genome library construction was facilitated by the helpful insight of Alina Gutu. Genomic analysis was carried out by Toshiro Ohsumi in the Department of Molecular Biology at Massachusetts General Hospital. Protein sequence analysis and identification of the tandem affinity purification protein complexes were provided by Ross Tomaino at the Taplin Mass Spectrometry Facility at Harvard Medical School, Boston, MA.
Footnotes
Citation Desbonnet C, Tait-Kamradt A, Garcia-Solache M, Dunman P, Coleman J, Arthur M, Rice LB. 2016. Involvement of the eukaryote-like kinase-phosphatase system and a protein that interacts with penicillin-binding protein 5 in emergence of cephalosporin resistance in cephalosporin-sensitive class A penicillin-binding protein mutants in Enterococcus faecium. mBio 7(2):e02188-15. doi:10.1128/mBio.02188-15.
REFERENCES
- 1.Williamson R, Calderwood SB, Moellering RC, Tomasz A. 1983. Studies on the mechanism of intrinsic resistance to β-lactam antibiotic in group D streptococci. J Gen Microbiol 129:813–822. doi: 10.1099/00221287-129-3-813. [DOI] [PubMed] [Google Scholar]
- 2.Rice LB, Carias LL, Rudin S, Hutton R, Marshall S, Hassan M, Josseaume N, Dubost L, Marie A, Arthur M. 2009. Role of class A penicillin-binding proteins in the expression of beta-lactam resistance in Enterococcus faecium. J Bacteriol 191:3649–3656. doi: 10.1128/JB.01834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice LB, Carias LL, Hutton-Thomas R, Sifaoui F, Gutmann L, Rudin SD. 2001. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob Agents Chemother 45:1480–1486. doi: 10.1128/AAC.45.5.1480-1486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbeloa A, Segal H, Hugonnet JE, Josseaume N, Dubost L, Brouard JP, Gutmann L, Mengin-Lecreulx D, Arthur M. 2004. Role of class A penicillin-binding proteins in PBP5-mediated beta-lactam resistance in Enterococcus faecalis. J Bacteriol 186:1221–1228. doi: 10.1128/JB.186.5.1221-1228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comenge Y, Quintiliani R Jr, Li L, Dubost L, Brouard JP, Hugonnet JE, Arthur M. 2003. The CroRS two-component regulatory system is required for intrinsic beta-lactam resistance in Enterococcus faecalis. J Bacteriol 185:7184–7192. doi: 10.1128/JB.185.24.7184-7192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kristich CJ, Little JL, Hall CL, Hoff JS. 2011. Reciprocal regulation of cephalosporin resistance in Enterococcus faecalis. mBio 2:e00199-11. doi: 10.1128/mBio.00199-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristich CJ, Wells CL, Dunny GM. 2007. A eukaryotic-type Ser/Thr kinase in Enterococcus faecalis mediates antimicrobial resistance and intestinal persistence. Proc Natl Acad Sci U S A 104:3508–3513. doi: 10.1073/pnas.0608742104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacco E, Cortes M, Josseaume N, Rice LB, Mainardi JL, Arthur M. 2014. Serine/threonine protein phosphatase-mediated control of the peptidoglycan cross-linking l,d-transpeptidase pathway in Enterococcus faecium. mBio 5:e01446-14. doi: 10.1128/mBio.01446-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesić D, Kristich CJ. 2012. MurAA is required for intrinsic cephalosporin resistance of Enterococcus faecalis. Antimicrob Agents Chemother 56:2443–2451. doi: 10.1128/AAC.05984-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnet S, Bellais S, Dubost L, Fourgeaud M, Mainardi JL, Petit-Frère S, Marie A, Mengin-Lecreulx D, Arthur M, Gutmann L. 2007. Identification of the l,d-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J Bacteriol 189:3927–3931. doi: 10.1128/JB.00084-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacco E, Hugonnet JE, Josseaume N, Cremniter J, Dubost L, Marie A, Patin D, Blanot D, Rice LB, Mainardi JL, Arthur M. 2010. Activation of the l,d-transpeptidation peptidoglycan cross-linking pathway by a metallo-d,d-carboxypeptidase in Enterococcus faecium. Mol Microbiol 75:874–885. doi: 10.1111/j.1365-2958.2009.07014.x. [DOI] [PubMed] [Google Scholar]
- 12.Pereira SF, Goss L, Dworkin J. 2011. Eukaryote-like serine/threonine kinases and phosphatases in bacteria. Microbiol Mol Biol Rev 75:192–212. doi: 10.1128/MMBR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Séraphin B. 1999. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol 17:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- 14.Hall CL, Tschannen M, Worthey EA, Kristich CJ. 2013. IreB, a Ser/Thr kinase substrate, influences antimicrobial resistance in Enterococcus faecalis. Antimicrob Agents Chemother 57:6179–6186. doi: 10.1128/AAC.01472-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice LB, Carias LL, Hutton-Thomas R, Rudin S. 2007. Interaction of related Tn916-like transposons: analysis of excision events promoted by Tn916 and Tn5386 integrases. J Bacteriol 189:3909–3917. doi: 10.1128/JB.00859-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliopoulos GM, Wennersten C, Moellering RC. 1982. Resistance to β-lactam antibiotics in Streptococcus faecium. Antimicrob Agents Chemother 22:295–301. doi: 10.1128/AAC.22.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otero LH, Rojas-Altuve A, Llarrull LI, Carrasco-López C, Kumarasiri M, Lastochkin E, Fishovitz J, Dawley M, Hesek D, Lee M, Johnson JW, Fisher JF, Chang M, Mobashery S, Hermoso JA. 2013. How allosteric control of Staphylococcus aureus penicillin binding protein 2a enables methicillin resistance and physiological function. Proc Natl Acad Sci U S A 110:16808–16813. doi: 10.1073/pnas.1300118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowman SK, Simon MD, Deaton AM, Tolstorukov M, Borowsky ML, Kingston RE. 2013. Multiplexed Illumina sequencing libraries from picogram quantities of DNA. BMC Genomics 14:466. doi: 10.1186/1471-2164-14-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam MM, Seemann T, Bulach DM, Gladman SL, Chen H, Haring V, Moore RJ, Ballard S, Grayson ML, Johnson PD, Howden BP, Stinear TP. 2012. Comparative analysis of the first complete Enterococcus faecium genome. J Bacteriol 194:2334–2341. doi: 10.1128/JB.00259-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poyart C, Trieu-Cuot P. 1997. A broad-host-range mobilizable shuttle vector for the construction of transcriptional fusions to beta-galactosidase in gram-positive bacteria. FEMS Microbiol Lett 156:193–198. doi: 10.1016/S0378-1097(97)00423-0. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal GK, Thelen JJ. 2005. Development of a simplified, economical polyacrylamide gel staining protocol for phosphoproteins. Proteomics 5:4684–4688. doi: 10.1002/pmic.200500021. [DOI] [PubMed] [Google Scholar]
- 22.Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, Wilm M, Séraphin B. 2001. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24:218–229. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- 23.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl MW. 2002. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stk protein sequences from CV598 and Cro1. Download
Extracellular domain of the eukaryote-like serine-threonine kinase from CV598, Cro1, and E. faecalis OG1RF. Download
Ceftriaxone disc assay of the Cro2 mutant. (Left) Cro2 mutant growing all the way up to the disc. (Right) Large zone around ceftriaxone disc in Cro2 mutant transformed with wild-type stpA-stk. Download
Extended maximum likelihood tree. P5AP homologues from Firmicutes. E. faecium Cro1 is highlighted in red. P5AP from E. faecium is closer to other enterococcal P5AP sequences (blue branches); the closely related Streptococcus, Leuconostoc, and Lactococcus are highly divergent from the Enterococcaceae, making the Lactobacillales lie in three separate clusters (light blue boxes). The Bacillales form all a single branch (light magenta box) with the exception of Staphylococcus (shown in a yellow box with an asterisk). The Clostridia (Clostridium butyricum and Clostridium pasteurianum) are shown in light green. Download
High-stringency mutations identified in 558CRO and 598CRO1 by next-generation sequencing.
Mass spectrometry peptide and protein data for TAP-purified protein complexes.
Distance matrices for phylogenetic analysis.
Gene expression data for CV571 with and without penicillin exposure.