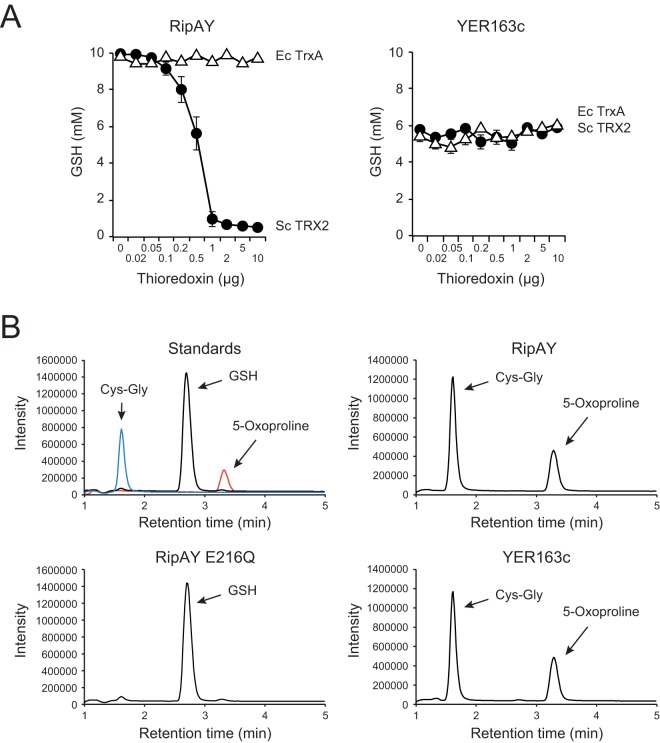
FIG 5 .
Activation of the GSH degradation activity of RipAY by purified recombinant thioredoxin. (A) Effect of purified recombinant thioredoxin on the GSH degradation activity of RipAY and YER163c. GSH (10 mM) was incubated with 1 µg of RipAY (left) and 2 µg of YER163c (right) in the presence of various amounts of yeast TRX2 (solid circles) and E. coli TrxA (open triangles) thioredoxins for 60 min at 30°C in 100 µl of 50 mM Tris-HCl (pH 8.0) reaction mixture. (B) RipAY exhibits γ-glutamyl cyclotransferase activity. GSH (1 mM) was incubated with 3 µg of RipAY (upper right panel), RipAY E216Q (bottom left panel), and YER163c (bottom right panel) for 60 min at 30°C in 100 µl of 50 mM Tris-HCl (pH 8.0) reaction mixture. For RipAY and RipAY E216Q, 2 µg of yeast TRX2 thioredoxin was added to the reaction mixture. The reaction mixture was then incubated at 95°C for 5 min to stop the reaction, and the substrate and the degradation products in the terminated sample were analyzed by LC-MS as described in Materials and Methods. The substrate and products in the reaction mixture were identified with authentic standards (upper left panel, with the individually detected three peaks shown together). GSH (m/z =308.3), 5-oxoproline (m/z = 130.1), and Cys-Gly (m/z =179.2) were detected at 2.71, 3.32, and 1.63 min, respectively.