Figure 2.
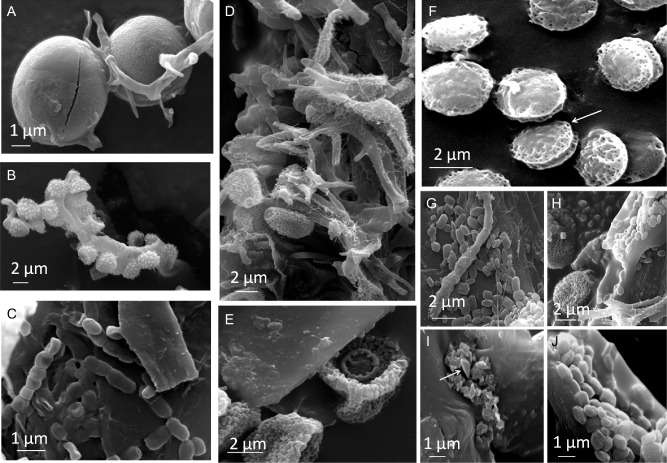
High vacuum, secondary electrons SEM images of fungal and bacterial structures. To obtain SEM imaging, micro‐samples were gently attached on stubs with carbon adhesive and analysed using a SEM instrument (EVO50, Carl‐Zeiss Electron Microscopy Group) equipped with a detector for secondary electrons (SE) at 20 keV. Samples were directly covered with gold with a Baltec Sputter Coater; the sputtering was performed under an Argon gas flow, at 50 mm working distance with 0.05 mbar of pressure and a current of 40 ma, for 60 s, to obtain a film of gold of about 15 nm.
A. Globular structures (fungal conidia or algae) wrapped in filaments similar to fungal hyphae and characterized by the presence of longitudinal openings similar to germination seams.
B. Clusters of echinated conidia not directly identifiable at the fungal genera level.
C. Chain of spores of less than 1 μm in diameter, presumably belonging to filamentous bacterial structures (Actinomycetales), attached to a cellulose fibre taken from the drawing.
D. Ellipsoidal conidia belonging to the fungal species E. halophilicum, collected from foxing spots on the verso of the drawing; the conidia present a significant variation in size (4–8/4–9 μm) that, in terms of shape, ornamentation and dimensions, are consistent with those of the anamorphous state of E. halophilicum, namely Aspergillus halophilicus.
E. Prominent scars in conidia were also pointed out.
F. Lenticular, rough, with shallow furrow and bordered by low ridges; ascospores attributable to E. halophilicum.
G–H. typical monoclinic prismatic monohydrate crystals of calcium oxalate attached to fungal structures and cellulose fibres sampled from the drawing.
I. Typical tetragonal (arrow) dihydrate calcium oxalate crystals attached to cellulose fibres.
J. Detail of prismatic monohydrate crystals of calcium oxalate embedded in a non‐defined matrix attached to the fungal structures, and cellulose fibres collected in foxing spots on the drawing.
