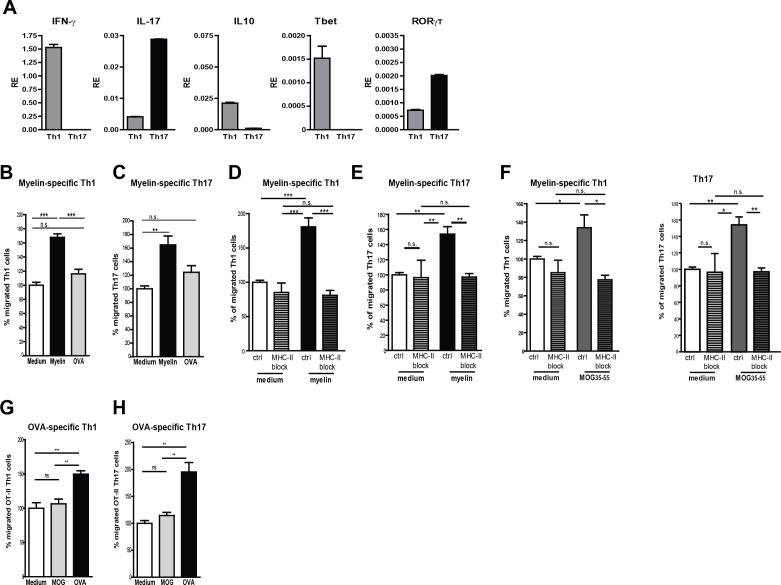
Figure 3. Migration of myelin-specific T-cells depends on presentation of myelin-antigens in MHC-II by BECs.
(A) Th1 and Th17 subsets were generated in-vitro from naive CD4+CD62Lhigh 2D2 T cells. Expression of IFN-γ, Il−17, IL−10, T-bet and RORγT was determined using qRT-PCR. Data are the means of triplicate values ± SEM of three independent experiments. (B) mBECs were seeded onto trans-wells, activated with TNFα and loaded with myelin for 24 hr. As a control, BECs loaded with the CNS-unrelated antigen OVA or unloaded BECs were used. (B) Th1 or (C) Th17 2D2 T-cells were added to the upper compartment and T-cell migration was quantified by flow cytometry 3 hr later using fluorescent labelled beads as reference. To block antigen recognition by T-cells, an MHC-II blocking antibody was added to mBECs one hour prior addition of the (D) Th1 or (E) Th17 cells. The MHC-II blocking antibody was present during the 3 hr incubation with the T-cells. (F) Transmigration of Th1 and Th17 cells over a monolayer of MOG35-55 pulse-loaded activated mBECs was analyzed using Transwells. Migration was assessed in the presence of an MHC-II blocking antibody or control antibody. *p<0.05, **p<0.01, ***p<0.001 (ANOVA with Bonferroni correction). The average frequency of T cells that transmigrated in the control setting are 10.8% ± 1.2 for 2D2 Th1 and 11.6% ± 0.4 for 2D2 Th17. (G,H) Th1 and Th17 subsets were generated in-vitro from naive CD4+CD62Lhigh OT-II T cells. mBECs were seeded onto trans-wells, activated with TNF and loaded with OVA for 24 hr. As a control, BECs loaded with MOG35-55 or unloaded BECs were used. Th1 (G) or Th17 (H) OT-II T-cells were added to the upper compartment and T-cell migration was quantified by flow cytometry 3 hr later using fluorescent labelled beads as reference. Average frequency of OT-II Th1 and OT-II Th17 that transmigrated in the control settings are 7.9% ± 1.9 and 12.5% ± 1.4, respectively.**p<0.01, ***p<0.001 (ANOVA with Bonferroni correction).