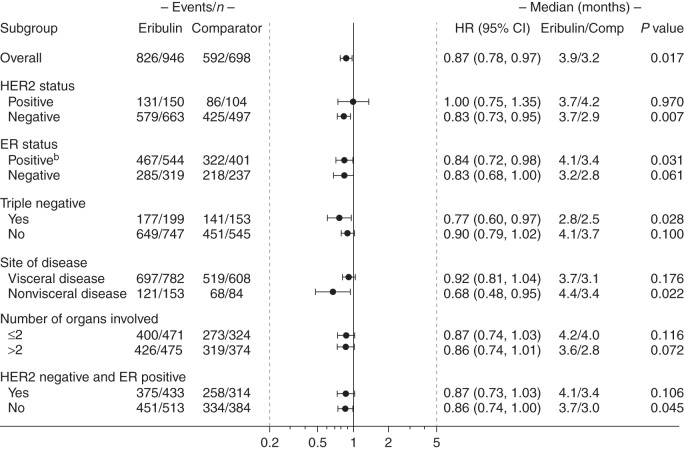
Figure 2.
PFS in patients who received eribulin according to the EU labela, based on investigator review. HR was estimated based on the Cox model without covariates, with stratification factors: study, region, HER2 status, and prior capecitabine use. For HER2 subgroup analysis, HER2 was not used as a stratification factor. P value is estimated based on the stratified log-rank test. aPatients with locally advanced or MBC who had received one or more prior chemotherapeutic regimens for advanced disease (including an anthracycline and a taxane in either the adjuvant or metastatic setting, unless patients were not suitable for these treatments). bA significant interaction between study and treatment was observed in this analysis when a treatment*study interaction term was used (P < 0.01; data not shown). CI, confidence interval; ER, estrogen receptor; EU, European Union; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; MBC, metastatic breast cancer; PFS, progression-free survival.