Abstract
Acute pancreatitis (AP) may cause significant persistent multi-organ dysfunction. Carvacrol (CAR) possesses a variety of biological and pharmacological properties. The aim of the present study was to analyze the hepatic protection of CAR on AP induced by cerulein and to explore the underlying mechanism using in vivo studies. The rats were randomized into groups to receive (1) no therapy; (2) 50 µg/kg cerulein at 1-h intervals by four intraperitoneal injection (i.p.); (3) 50, 100 and 200 mg/kg CAR by one i.p.; and (4) cerulein + CAR after 2 h of cerulein injection. 12 h later, serum was provided to assess the blood AST, ALT and LDH values. Also, liver tissues were obtained for histological and biochemical measurements. Liver oxidative stress markers were evaluated by changes in the amount of lipid peroxides measured as MDA and changes in tissue antioxidant enzyme levels, SOD, CAT and GSH-Px. Histopathological examination was performed using scoring systems. Oxidative damage to DNA was quantitated in studied tissues of experimental animals by measuring the increase in 8-hydroxydeoxyguanosine (8-OHdG) formations. We found that the increasing doses of CAR decreased pancreatitis-induced MDA and 8-OH-dG levels. Moreover, the liver SOD, CAT and GSH-Px activities in the AP + CAR group were higher than that of the rats in the AP group. In the treatment groups, AST, ALT and LDH were reduced. Besides, necrosis, coagulation and inflammation in the liver were alleviated (p < 0.05). We suggest that CAR could be a safe and potent new drug candidate for treating AP through its antioxidative mechanism of action for the treatment of a wide range of disorders related to hepatic dysfunction.
Keywords: Experimental acute pancreatitis, Liver damage, Carvacrol, Antioxidant response, Oxidative DNA damage, Histopathology
Introduction
Acute pancreatitis (AP) is a unique acute inflammatory disease of the pancreas that may also involve peripancreatic tissues and even remote organs (Yang et al. 2014). The pathophysiological mechanism of AP is characterized by the inflammatory response, multiple organ dysfunction syndrome (MODS), and even death (Liang et al. 2014). Persistent oxidative stress and excess LPO are induced by inflammatory processes in a self-perpetuating process and cause progressive accumulation of DNA damage in target organs. Together with deregulation of cell homeostasis, the resulting genetic changes act as driving force in inflammation-associated human disease pathogenesis (Sawa and Ohshima 2006).
The production and importance of essential oils (EOs) have steadily increased during the last decade (Turkez et al. 2012a). This increment is likely due to a wide spectrum of antimicrobial properties (Rocha-Guzmán et al. 2007). Carvacrol (CAR) used as the most active constituent of thyme EOs, exhibits antifungal, antiviral, antitumor and anti-inflammatory activities. It is also used as flavor agent in cosmetic and food products (Adorjan and Buchbauer 2010). A particularly interesting feature of CAR has recently come to light. It acts as antioxidant, free radical scavenger and anti-lipid peroxidative agent (Elhabazi et al. 2006; Beena and Rawat 2013; Aydin et al. 2014). Natural antioxidants or antioxidant featured synthetic compounds have been found to protect various organs from oxidative injuries (Cingolani et al. 2000; Cacciatore et al. 2003; Rispoli et al. 2004).
There is now support that reactive oxygen species (ROS) produced as a result of oxidative stress may be involved in etiopathogenesis of AP (Liang et al. 2014). The precise role of ROS in the pathogenesis of AP is a matter of ongoing debate. The results underscore the important function of ROS-producing cells in the progression of the experimental AP and suggest these cells as a specific target for future studies with antioxidants (Müller et al. 2014). Persistent organ failure is major determinant of mortality in AP (Turkvatan et al. 2014). Liver tissue is susceptible to oxidative stress because of its high oxygen consumption and modest antioxidant defenses (Shiba et al. 2014). Marked microcirculatory disorders and degenerative changes in the liver reflect the nature of the pathological process in AP (Nepomnyashchikh et al. 2013). The effects of free radicals result in adverse alterations on the structure and function of cell membrane system, contributing to subsequent cell damage (Inkielewicz-Stepniak et al. 2014). Moreover, these free radicals also attack antioxidant defense system, leading to the loss of antioxidant components such as SOD, CAT, and GSH-Px (Cheshchevik et al. 2012). Many of the drugs prescribed in pharmacies routinely are suspected of causing AP. In addition, they pose a high risk with cumulative doses (Giorda et al. 2014; Oliveira et al. 2014).
Rationally, it is time to look for alternative truly effective interventions for patients with, a dreadful disease, AP. Since antioxidants are safe and tolerable, and have shown to be effective in some recent clinical trials (Cacciatore et al. 2005; Heuking et al. 2009; Larusch et al. 2014), using biochemical and histopathological methods we suggest for the first time that CAR can be used as an antioxidant therapy in an experimental model of cerulein-injection induced pancreatitis. In addition, we explore the underlying mechanism of AP-induced liver damages.
Materials and methods
Animals
Seventy adult male Spraque-Dawley rats (weighing 200–250 g) obtained from Medical Experimental Application and Research Center, Atatürk University, were used for this study. Animals were housed inside polycarbonate cages in an air-conditioned room (22 ± 2 °C) with 12-h light–dark cycle. Standard rat feed and water were provided ad libitum. The rats were allowed to acclimatize to the laboratory environment for 7 days before the start of the experiment. All procedures were performed in conformity with the Institutional Ethical Committee for Animal Care and Use at the Atatürk University (protocol number: B.30.2.ATA.0.23.85-11) and the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Experimental protocols
Animals were randomly divided into eight groups (n = 7, each): (1) vehicle-treated group (control); (2) AP group; (3, 4, 5) CAR-treated groups (at three different dose); (6, 7, 8) CAR-treated AP groups.
AP was induced by cerulein (Sigma-Aldrich, GmbH, Steinheim, Germany) administered i.p. four times with 1 h intervals at a dose of 50 µg/kg b.w. AP was assessed after last injection of cerulein by measurement of serum amylase and lipase levels. Animals without induction of acute pancreatitis (control) were treated i.p. with saline at the same time as animals were treated with cerulein.
To evaluate the effects of CAR, animals were treated with carvacrol in 10 mL of saline (Peptide International Inc, Osaka, Japan). The CAR groups received one i.p. injection of 50, 100 and 200 mg/kg b.w. Therapeutic treatments were administered after 2 h of cerulein injection. The rats were anesthetized with isoflurane after 12 h taking CAR and euthanized by exsanguination with blood retained for serum harvest. These investigations are based on the works published by Hagiwara et al. (2009) and Yu et al. (2012).
Biochemical analyses
Amylase and lipase measurement
Serum amylase and lipase levels were determined spectrophotometrically using an automated analyzer (Olympus AU 600, Diamond Diagnostic, Holliston, MA, USA). All chemicals were obtained from Sigma (St. Louis, MO, USA).
Determination of lipid peroxidation
Lipid peroxidation was determined by quantifying malondialdehyde (MDA) concentrations, which was spectrophotometrically measured by the absorbance of a red-colored product with thiobarbituric acid (Ohkawa et al. 1979).
Antioxidant enzymes
The activities of antioxidant enzymes were assayed in liver tissue of each group. For this purpose, the tissues were removed, cleaned, and washed in ice-cold normal saline for biochemical studies. All samples were stored at −70 °C until assayed. The 10 % homogenates of tissues were prepared in a phosphate buffer (0.1 M, pH 7.4) containing 1 mmol ethylenediaminetetra acetic acid (EDTA), 0.25 mM sucrose, 10 mM potassium chloride (KCl), and 1 mM phenylmethyl sulfonyl fluoride (PMSF).
The superoxide dismutase (SOD) activity was determined by the method of Misra and Fridovich (1972). In this test, the degree of inhibition of pyrogallol oxidation by liver homogenate supernatant was measured. The change in absorbance was read at 470 nm against blank every 3 min on a spectrophotometer and the enzyme activity was expressed as 50 % inhibition of adrenaline oxidation/min.
The catalase (CAT) activity was measured as follows (Beers and Sizer 1952). For CAT activity, dichromatic acetic acid is reduced to chromic acetate when heated in the presence of H2O2, with the formation of perchloric acid as an unstable intermediate. In the test, the green color development was read at 590 nm against blank in a spectrophotometer. The activity of CAT was expressed as μmole of H2O2 consumed/mg protein/min.
The glutathione peroxidase (GSH-Px) activity was determined essentially as described by Rotruck et al. (1973). The rate of glutathione oxidation by H2O2, as catalyzed by the GSH-Px present in the supernatant was determined and the color developed was read against a reagent blank at 412 nm in a spectrophotometer. In the test, the enzyme activity was expressed as μmole of glutathione oxidized/mg protein/min.
Liver function assessment
Blood samples were collected into serum superetor tubes (Microtainer; Becton–Dickinson, Franklin Lakes, NJ, USA), allowed to stand (75–90 min), and centrifuged (11.000g, 5 min). Serum was harvested and stored at −20 °C. The alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) enzyme activities were measured for assessing liver injuries by an automated biochemical analyzer (Olympus AU 2700) with commercially available testing kits (Bioclinica, Audubon, PA, USA).
Determination of 8-OH-dG level
8-hydroxy-2′-deoxyguanosine assay kits were purchased from Cayman Chemical Company (Ann Arbor, MI, USA) for determining 8-OH-dG levels in the liver samples. Since it is a competitive assay that can be used for the quantification of 8-OH-dG in homogenates and recognizes both free 8-OHdG and DNA-incorporated 8-OH-dG, many studies are performed to use this protocol. This assay depends on the competition between 8-OH-dG and 8-OH-dG-acetylcholinesterase (AChE) conjugate (8-OH-dGTracer) for a limited amount of 8-OH-dG monoclonal antibody (Abdel-Wahab and Metwally 2011). All procedures were carried out in accordance with the provider’s manual.
Histopathological examination
The liver tissues of rats were fixed in buffered 10 % formalin solution for 24 h and embedded in paraffin wax. Tissues were then sectioned at 5-μm, stained with hematoxylin eosin (H&E). The PAS, Reticulin and Oil Red-O staining kit steps were performed in accordance with the manufacturer’s instructions (Sigma-Aldrich, Inc., St. Louis, MO, USA). A semi quantitative evaluation of liver tissue was accomplished by scoring the degree of severity according to the formerly published criteria (Teixeira et al. 1982). For each liver section, one whole slide was examined for hepatic damage, intercellular fibrous, hepatic glycogen and lipid content were observed under bright field using an Olympus BX60 microscope equipped with a digital CCD. In addition, high-resolution pictures (×200) of samples were taken under the same microscope. Hepatic damage was scored with maximum score of 18. The maximum score for hepatic glycogen, lipid content and intercellular fibrous material was 3. A blind evaluation of histological samples was performed by expert investigators in reading histological slides.
Statistical analysis
For statistical analysis, we used SPSS for Windows 18.0 (SPSS Inc., Chicago, IL, USA). The obtained biochemical data were analyzed using one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) and Duncan’s Multiple Range post hoc tests for multiple comparisons of control and experimental groups. We checked whether the data had normal distribution by using Kolmogorov Smirnow test. Differences between nonparametric groups in the scores for histopathological findings were examined by the Mann–Whitney U test. Results are presented as mean ± standard deviation (SD) of seven repetitions. And values p < 0.05 were regarded as statistically significant.
Results
Tables 1 and 2 show the effects of CAR on biochemical parameters in all experimental groups. The serum amylase levels in cerulein-induced AP were increased from an average 490 U/L to about 2350 U/L as compared with those of control rats. Similarly, lipase level was increased from 25 to 127 U/L. Following intraperitoneal injection of CAR alone, the amylase and lipase levels were not changed. Moreover, in animals with AP, the increasing dosages of CAR showed positive effects on above parameters and the values were significantly decreased with respect to high dose of CAR (p < 0.05).
Table 1.
Effects of CAR treatments on serum amylase and lipase levels in cerulein-induced AP
| Groups | Amylase (U/L) | Lipase (U/L) |
|---|---|---|
| Control | 490.03 ± 17.43 | 25.95 ± 1.65 |
| AP | 2350.60 ± 398.29*a | 127.28 ± 7.19*a |
| CAR 50 mg/kg | 504.72 ± 19.89b | 26.05 ± 2.36b |
| CAR 100 mg/kg | 476.41 ± 31.69b | 24.01 ± 3.08b |
| CAR 200 mg/kg | 482.48 ± 19.61b | 24.48 ± 1.16b |
| AP + CAR 50 mg/kg | 1722.20 ± 402.15*a | 115.19 ± 4.22*a |
| AP + CAR 100 mg/kg | 1301.14 ± 329.14*a | 91.34 ± 8.56*a |
| AP + CAR 200 mg/kg | 699.72 ± 38.76b | 38.21 ± 4.60b |
Data are presented as mean ± SD (n = 7)
* Symbole < 0.05 represents significant difference among the groups compared to controls. p < 0.05, significantly different from a Control group
bAP group by Duncan’s Multiple Range post hoc tests for multiple comparisons of control and experimental groups
Table 2.
Effect of CAR treatment on liver SOD, CAT, and GSH-Px activities and MDA levels in cerulein-induced AP
| Groups | SOD (U/mg protein) | CAT (U/mg protein) | GPx (U/mg protein) | MDA (nmol/mg protein) |
|---|---|---|---|---|
| Control | 31.09 ± 0.17 | 3.02 ± 0.27 | 1.01 ± 0.13 | 6.95 ± 0.90 |
| AP | 17.45 ± 0.12*a | 0.52 ± 0.22*a | 0.22 ± 0.10*a | 15.25 ± 0.30*a |
| CAR 50 mg/kg | 32.31 ± 1.18b | 3.17 ± 0.20b | 1.20 ± 0.17b | 6.90 ± 0.10b |
| CAR 100 mg/kg | 32.80 ± 1.30b | 3.67 ± 0.26b | 1.25 ± 0.21b | 6.87 ± 0.16b |
| CAR 200 mg/kg | 35.40 ± 1.44b | 4.43 ± 0.25b | 1.57 ± 0.09b | 6.89 ± 0.24b |
| AP + CAR 50 mg/kg | 17.91 ± 0.31*a | 1.05 ± 0.32*a | 0.55 ± 0.12*a | 14.96 ± 0.12*a |
| AP + CAR 100 mg/kg | 18.24 ± 0.42*a | 1.59 ± 0.28*a | 0.60 ± 0.09*a | 14.41 ± 0.26*a |
| AP + CAR 200 mg/kg | 30.87 ± 0.89b | 2.91 ± 0.17b | 0.92 ± 0.11b | 7.93 ± 0.15b |
Data are presented as mean ± SD (n = 7)
* Symbole < 0.05 represents significant difference among the groups compared to controls. p < 0.05, significantly different from a Control group
bAP group by Duncan’s Multiple Range post hoc tests for multiple comparisons of control and experimental groups
As presented in Table 2, the SOD, CAT, and GSH-Px activities were markedly decreased in liver of AP rats while MDA increased (p < 0.05) compared to those found in the controls. CAR groups alone showed the increased levels of antioxidant enzymes at both dosage (50 and 100 mg/kg) but the best result was observed at a dose of 200 mg/kg of CAR. In the AP + CAR group at high dose, the antioxidant enzymes revealed statistically significant increases (p < 0.05) and oxidative stress returned to the control levels.
In the present study, activity levels of serum marker enzymes of liver are found markedly elevated in rats with AP (Table 3). No such changes were observed in control rat samples. As is evident from Table 3, CAR doses alone (50, 100 and 200 mg/kg) did not change the activity levels of AST, ALT and LDH (p < 0.05). In addition, the treatment with CAR can bring a decrease in the activity levels of these enzymes when compared to AP. Our results clearly revealed that CAR presented a partial positive effect on the activities of the enzymes without depending on dose against AP-induced liver damages. The levels of ALT (50 mg/kg CAR), ALT and AST (100 mg/kg CAR) enzyme samples showed a significant similarity with control values after treatments with CAR in AP. Moreover, the LDH enzyme activity was normalized by doses of CAR exposure (200 mg/kg).
Table 3.
Effect of CAR treatment on liver AST, ALT, and LDH activities in cerulein-induced AP
| Groups | AST(U/L) | ALT(U/L) | LDH (U/L) |
|---|---|---|---|
| Control | 179.38 ± 12.01 | 52.61 ± 2.29 | 4059.35 ± 404.72 |
| AP | 294.62 ± 15.22*a | 77.85 ± 7.07*a | 7622.04 ± 812.37*a |
| CAR 50 mg/kg | 178.46 ± 6.48b | 53.21 ± 3.14b | 4043.17 ± 371.25b |
| CAR 100 mg/kg | 178.87 ± 9.13b | 51.25 ± 5.12b | 4052.11 ± 463.32b |
| CAR 200 mg/kg | 180.91 ± 8.94b | 52.38 ± 1.36b | 4061.41 ± 570.49b |
| AP + CAR 50 mg/kg | 268.46 ± 13.47*a | 70.19 ± 2.15b | 6986.72 ± 643.43*a |
| AP + CAR 100 mg/kg | 208.01 ± 11.29*a | 65.76 ± 2.07b | 5947.52 ± 701.19*a |
| AP + CAR 200 mg/kg | 184.19 ± 9.84b | 53.07 ± 3.22b | 4089.81 ± 298.68b |
Data are presented as mean ± SD (n = 7)
* Symbole < 0.05 represents significant difference among the groups compared to controls. p < 0.05, significantly different from a Control group
bAP group by Duncan’s Multiple Range post hoc tests for multiple comparisons of control and experimental groups
The levels of 8-OH-dG, a hallmark of oxidative stress-DNA base damage, were measured using an 8-OH-dG detection kit. There were no significant difference between the levels of 8-OH-dG in the control and all CAR treated groups (Table 4). On the contrary, the level of 8-OH-dG was significantly higher in AP as compared to the control group. But co-treatment of CAR decreased the 8-OH-dG levels that were increased by cerulein-induced AP in a clear dose dependent manner.
Table 4.
Effect of CAR treatment on liver 8-OH-dG levels in cerulein-induced AP
| Groups | 8-OH-dG level (as pg/mL) |
|---|---|
| Control | 1.10 ± 0.10 |
| AP | 3.95 ± 0.42*a |
| CAR 50 mg/kg | 0.90 ± 0.09b |
| CAR 100 mg/kg | 1.15 ± 0.10b |
| CAR 200 mg/kg | 1.08 ± 0.12b |
| AP + CAR 50 mg/kg | 2.25 ± 0.22*a |
| AP + CAR 100 mg/kg | 1.55 ± 0.14*a |
| AP + CAR 200 mg/kg | 1.15 ± 0.18b |
Data are presented as mean ± SD (n = 7)
* Symbole < 0.05 represents significant difference among the groups compared to the controls. p < 0.05, significantly different from the a Control group
bAP group by Duncan’s Multiple Range post hoc tests for multiple comparisons of the control and the experimental groups
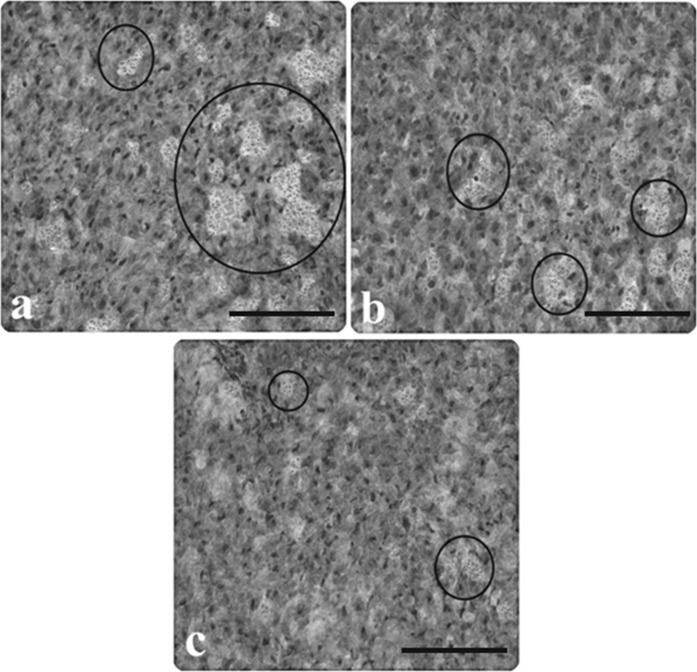
An in-depth characterization of hepatic pathology in AP rats, selected images of AP liver stained with different methods are presented in the figures. In H&E staining, the microscopic observations showed a significant congestion, infiltration, sinusoidal dilatation, vacuolization, tissue necrosis and edema in comparison to the controls (Figs. 1a, b, 2a–e). PAS staining clearly revealed depletion of glycogen in hepatocytes and also nonhomogeneous population of hepatocytes in AP-induced rats (Fig. 3a–c). Reticulin (Fig. 4a–c), and Oil Red O (Fig. 5a, b) showed increased intensity of fibrosis in portal veins and darkly stained hepatocytes indicating accumulation of lipid, respectively.
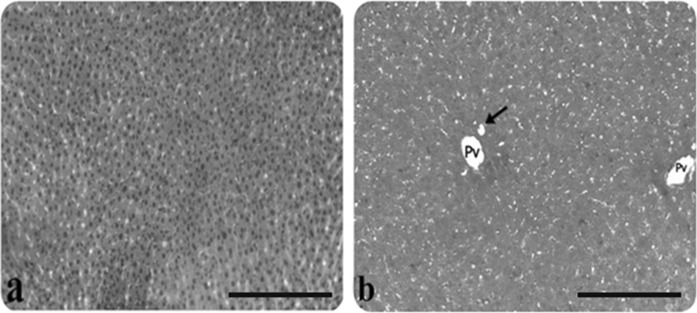
Fig. 1.
Light microscopic appearance of liver from control rats. a Hepatocytes, b portal vein (Pv), bile duct (arrow), (H&E staining). Scale bars in a, b 50 µm
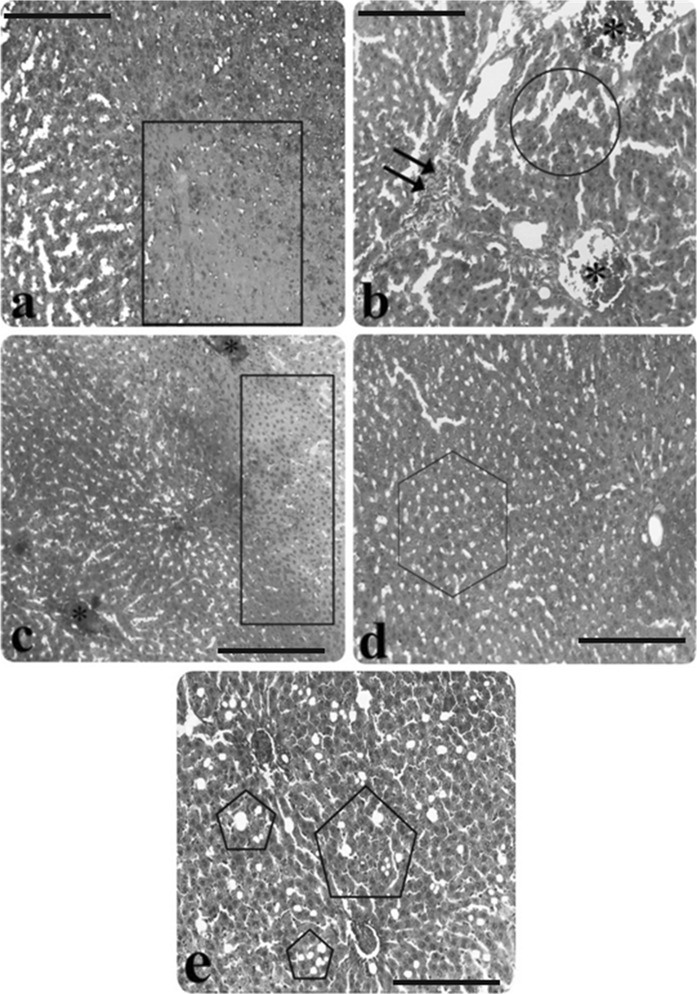
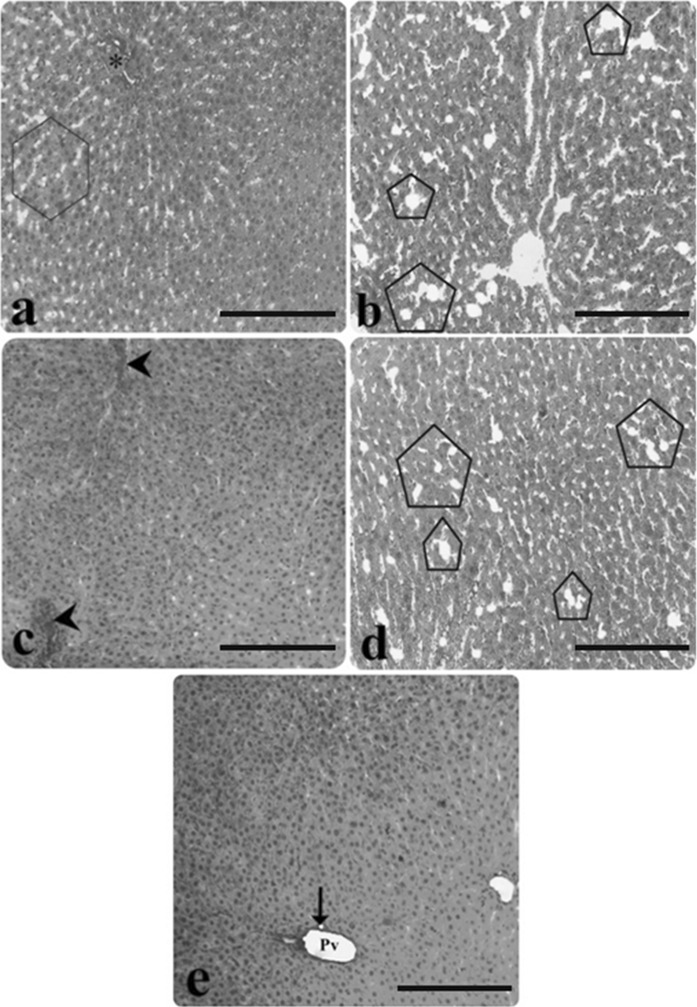
Fig. 2.
Light microscopic appearance of liver from AP group rats. a Hepatocyte necrosis (inside of the square symbol), b) Infiltration (double arrow), congestion (asterisk), sinusoidal dilatation (inside of the circle symbol), c hepatocyte necrosis (inside of the rectangular symbol), congestion (asterisk), d vacuolisation (inside of the hexagon symbol), e edema (inside of the pentagon symbol), (H&E staining). Scale bars in a, b, c, d, e 50 µm
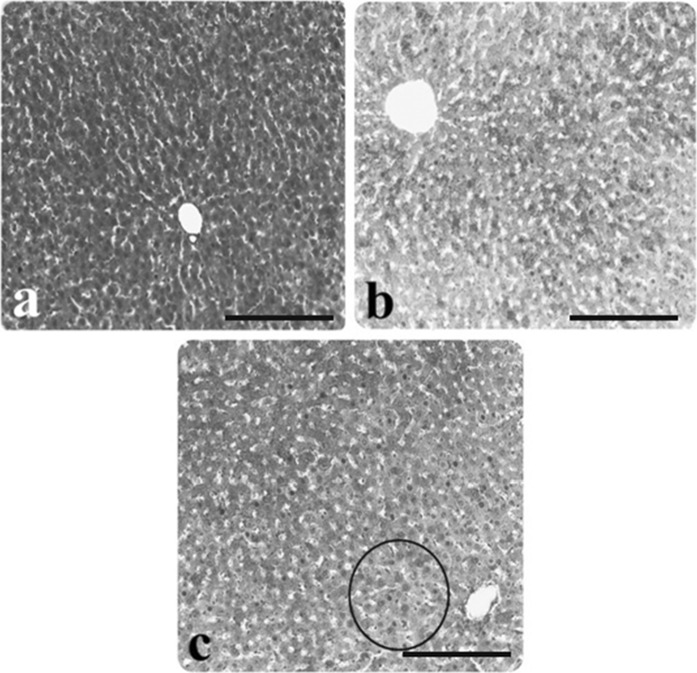
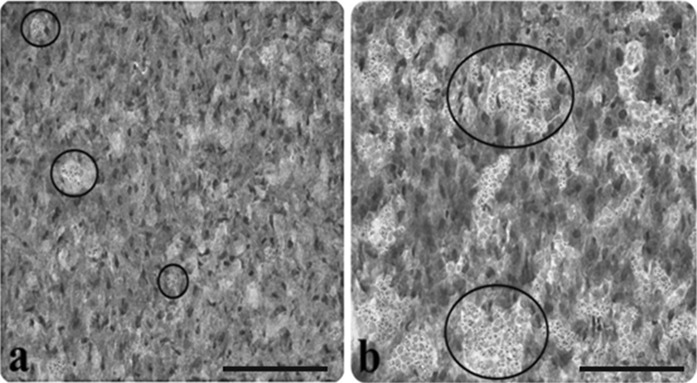
Fig. 3.
Light microscopic appearance of liver from control and AP group rats. a Glycogen stored in hepatocytes of the control rats, b slightly stained hepatocytes in rats with AP, c darkly stained hepatocytes in rats with AP (inside of the circle symbol), (PAS staining). Scale bars in a, b, c 50 µm
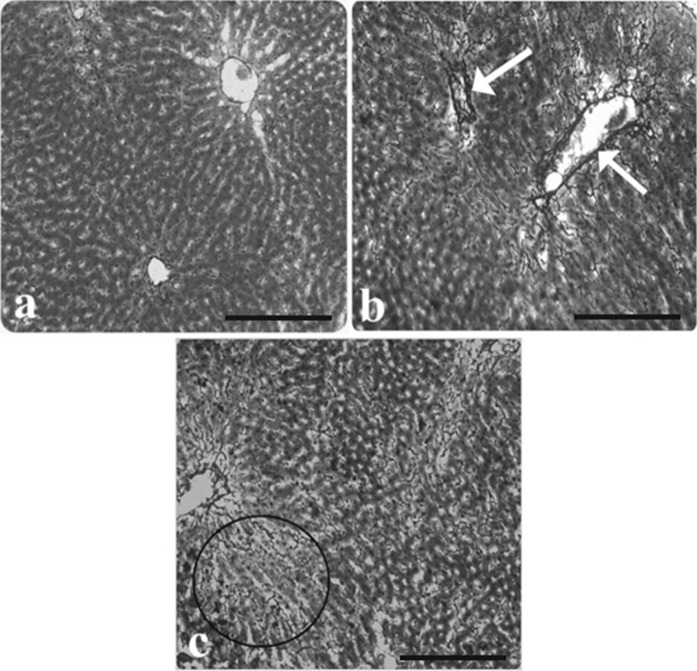
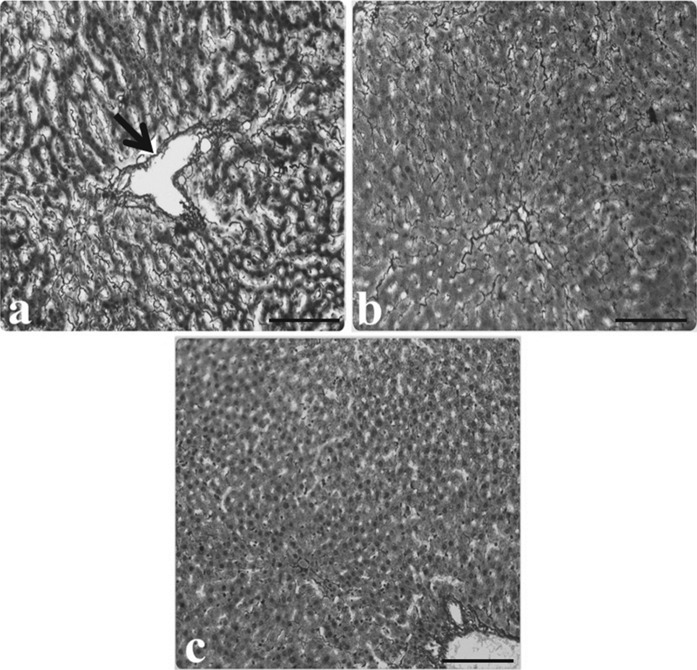
Fig. 4.
Light microscopic appearance of liver from control and AP group rats. a The normal architecture of liver from control rats, b increased intensity of fibrosis in portal veins of AP group rat liver (arrows), c increased fibrosis in intercellular space (inside of the circle symbol), (Reticulin-staining). Scale bars in a, b, c 50 µm
Fig. 5.
Light microscopic appearance of liver from control and AP group rats. a Lipid droplets in control group liver (inside of the circle symbols), b increasing accumulation of lipid in hepatocytes of the AP group, (Oil Red O staining). Scale bars in a, b 50 µm
Examination of liver sections in CAR groups revealed that the liver tissue retained its normal architecture (at 50, 100 and 200 mg/kg) (data not shown). Moreover, the pathological findings in the AP group were attenuated after treatments with CAR (Fig. 6a–e). CAR increased glycogen content of hepatocytes (Fig. 7a–c) and decreased intensity of fibrosis in the liver of the AP-animals (Fig. 8a–c). Furthermore, abnormal lipid accumulations in cells were not observed (Fig. 9a–c) and these protective effects were significantly associated with increased doses of CAR. After treatment with 200 mg/kg CAR liver tissue showed a normal structure and orderly arrangement and resembled those of control rats.
Fig. 6.
Light microscopic appearance of liver tissue following CAR exposure in AP rats. a Decreased vacuolisation in the AP + 50 mg/kg CAR group (inside of the hexagon symbol), and congestion (asterisk), b decreased vacuolisation in the AP + 50 mg/kg CAR group (inside of the pentagon symbols), c decreased congestion in the AP + 100 mg/kg CAR group (arrow heads), d decreased edema in the AP + 100 mg/kg CAR group as compared the AP + 50 mg/kg CAR group (inside of the pentagon symbols), e normal histological structure of liver in the AP + 200 mg/kg CAR group, portal vein (Pv), bile duct (arrow), (H&E staining). Scale bars in a, b, c, d, e 50 µm
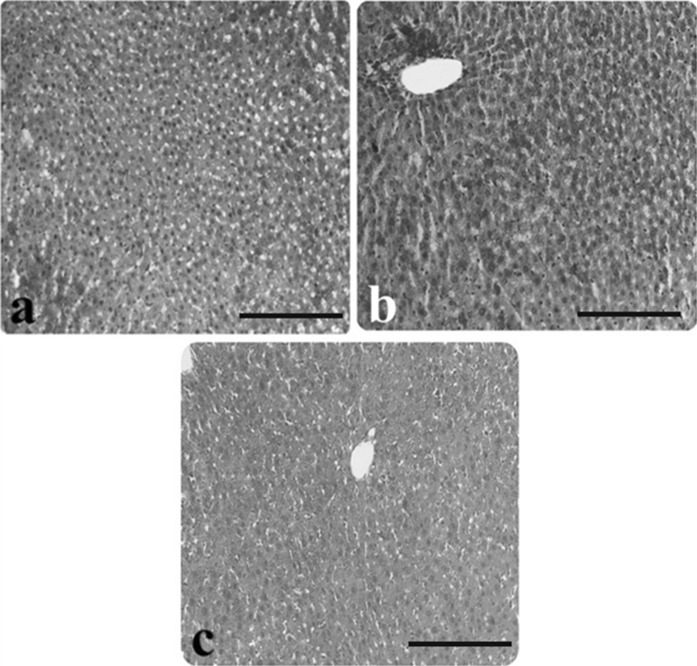
Fig. 7.
Light microscopic appearance of liver tissue following CAR exposure in AP rats. a AP + 50 mg/kg the CAR group, b increased glycogen content in the liver of the AP + 50 mg/kg CAR group, c liver histology similar to controls in the AP + 50 mg/kg CAR group, (PAS staining). Scale bars in a, b, c 50 µm
Fig. 8.
Light microscopic appearance of liver tissue following CAR exposure in AP rats. a Fibrosis in portal vein in the AP + 50 mg/kg CAR group (arrow), b decreased fibrosis in the AP + 100 mg/kg CAR group, c the normal architecture similar to controls in the AP + 200 mg/kg CAR group (Reticulin staining). Scale bars in a, b, c 50 µm
Fig. 9.
Light microscopic appearance of liver tissue following CAR exposure in AP rats. a Lipid droplets in hepatocytes of the AP + 50 mg/kg CAR group, b decreased lipid content in the AP + 100 mg/kg CAR group, c lipid content similar to controls in the AP + 200 mg/kg CAR group. The symbols are as Fig. 5a, b. (Oil Red O staining). Scale bars in a, b, c 50 µm
Histopathological scores of the groups are summarized in Table 5. The degree of pathological findings showed a significant difference between groups treated with cerulein and cerulein + CAR (p < 0.05).
Table 5.
Histopathological scores of liver pathology in cerulein-induced AP
| Groups | Scores for hepatic damage (maximum score of 18) | Scores for hepatic glycogen content (maximum score of 3) | Scores for hepatic lipid content (maximum score of 3) | Scores for intercellular fibrous material (maximum score of 3) |
|---|---|---|---|---|
| Control | 0.60 ± 0.12 | 2.54 ± 0.16 | 0.53 ± 0.17 | 0.42 ± 0.07 |
| AP | 9.30 ± 0.48*a | 0.59 ± 0.13*a | 2.61 ± 0.31*a | 2.47 ± 0.14*a |
| CAR 50 mg/kg | 0.57 ± 0.15b | 2.61 ± 0.21b | 0.52 ± 0.13b | 0.52 ± 0.13b |
| CAR 100 mg/kg | 0.61 ± 0.06b | 2.55 ± 0.11b | 0.53 ± 0.22b | 0.44 ± 0.09b |
| CAR 200 mg/kg | 0.59 ± 0.10b | 2.63 ± 0.18b | 0.50 ± 0.29b | 0.51 ± 0.10b |
| AP + CAR 50 mg/kg | 8.65 ± 0.32*a | 0.97 ± 0.11*a | 2.04 ± 0.10*a | 2.09 ± 0.24*a |
| AP + CAR 100 mg/kg | 7.60 ± 0.40*a | 1.43 ± 0.16*a | 1.54 ± 0.25*a | 1.75 ± 0.11*a |
| AP + CAR 200 mg/kg | 1.10 ± 0.24b | 2.47 ± 0.27b | 0.57 ± 0.18b | 0.63 ± 0.07b |
Data are presented as mean ± SD (n = 7)
* Symbole < 0.05 represents significant difference among the groups compared to the controls. p < 0.05, significantly different from the a Control group
bAP group. For abbreviations see legend in Table 1
Discussion
In the present study, we present CAR, the expression of which has not previously been described in hepatic tissue. The fact that acute relapsing pancreatitis can progress to chronic pancreatitis, which can further progress to pancreatic cancer, supports the importance of developing new potent and safe drugs to prevent and cure pancreatitis. Because free radicals and the ensuing inflammation are principally involved in AP progression, we hypothesized that CAR would render excellent protection against AP through its potent ability to inhibit oxidative stress and inflammation. Natural antioxidants are capable of inhibiting the ROS production and thereby reducing the associated intracellular oxidative stress (Leung and Chan 2009; Turkez et al. 2012a). Our current experimental study results successfully supported the hypothesis.
The correlation between pharmacodynamics and protection of cell integrity shows that CAR exhibits high antioxidant property and it can protect liver of rats with AP. Our results showed that cerulein administration induced a significant hemorrhage, infiltration, sinusoidal dilatation, vacuolization, tissue necrosis, and edema, increased intensity of fibrosis in portal veins, lipid accumulation and depletion of glycogen in hepatocytes. The elevated histopathological score of liver indicated that liver injury deteriorated following cerulein administration. The authors reported that these pathological features are specific for AP and cerulein-induced AP is one of the best known and widely used experimental models (Nepomnyashchikh et al. 2013). Indeed, ROS act as inflammatory mediator in this model so it was used in the current study. In this model, cerulein causes liver complications with extensive lipid peroxidation, producing some metabolites such as MDA (Lee et al. 2012). Cerulein can also cause systemic diseases in organs such as the lungs, heart, kidneys, and liver as well (Korkmaz et al. 2014). In our study, lipid peroxidation was elevated in the liver of rat after exposure to cerulein, as evidenced by increased MDA production. The presented pathological findings support the participation of free radical-induced oxidative cell injury in mediating cerulein. And MDA concentration provides direct evidence of the toxic processes caused by free radicals (Hashemipour et al. 2013).
Living cells have several mechanisms to restore their original redox state after a temporary exposure to increased levels of ROS (Geyikoglu et al. 2005; Geyikoglu and Turkez 2005, 2008). The main mechanism of redox homeostasis is based on redox-sensitive signaling cascades that lead to augmented expression of antioxidant enzymes (Droge 2002; Turkez et al. 2010). The results of this study are consistent with this case, in which the increase in MDA production was detected in cells exposed to cerulein, and this increase seems to have been sufficient to activate the genes responsible for the production of antioxidant enzymes after CAR treatment. In the present study, the treatment with CAR shows increased activity of antioxidant enzymes compared to cerulein treated animals indicating the potentiality of CAR to act as an antioxidant by preventing the peroxidative damage by cerulein. Furthermore, our results indicated that CAR treatment mitigated the oxidative DNA damages induced by cerulein. Lipid peroxidation can be prevented with induction of SOD, CAT and GSH-Px activities in the groups of rats treated with CAR. The decrease in the MDA level in the groups of rats treated with increasing CAR doses (especially 200 mg/kg) may be a significant indicator of an increase in the enzymatic and nonenzymatic antioxidants of defense mechanisms. As a matter of fact, CAR displays a concentration dependent antioxidant activity (Undeger et al. 2009). Surely, the high content of CAR could be reckoned as an important property that affects the antioxidant capacity (Saei-Dehkordi et al. 2010). Targets of accumulating ROS include proteins involved in antioxidant response.
The liver contains a number of endogenous antioxidants, to restrict steady state ROS levels (Turkez et al. 2012b). The balance between ROS generation and their elimination by endogenous antioxidant mechanisms play a critical role in preserving liver function; inappropriate levels of ROS likely precipitate impairment of liver function and abnormalities in liver structure (Perez-Gutierrez and Damian-Guzman 2012). In the conditions of AP, CAR shows effective properties for its pharmacological usage in liver. In accordance with our findings, CAR exerts greater levels of SOD and GSH-Px in the rat brain than the untreated controls (Youdim and Deans 2000). In the respiratory system, CAR treatment decreases MDA and shows a significant protective role (Boskabady and Jalali 2013). CAR also prevents lipid peroxidation, cell damages, and protects the antioxidant system in diethylnitrosamine-induced liver cancer in male Wistar albino rats (Jayakumar et al. 2012). It has also been reported that isoprenoids such as CAR, via post-transcriptional actions, suppress 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase activity in liver, the rate limiting enzyme for the synthesis of MDA (Akkol et al. 2012).
Elevated AST, ALT and LDH levels are another marker for AP disease processes (Tasic et al. 2014). The increased activities of ALT, AST and LDH in serum is mainly due to the leakage of these enzymes largely from the liver cytosol into the blood stream (El-Demerdash et al. 2005), which gives an indication of the abnormal function of liver. CAR appears to be effective in reducing the injurious effect of cerulein, as observed in the study. CAR is an indication for stabilization of plasma membrane, as well as repair of hepatic tissue damaged by cerulein. In fact, CAR was shown to be the effective antioxidant in protecting the d-galactosamine-induced lipid peroxidation of erythrocyte, liver and kidney membranes (Aristatile et al. 2009). The hydrophobic character of CAR and its mode of action suggest interaction with the cell membrane, where it dissolves in the phospholipid bilayer and is assumed to align between the fatty acid chains (Ayari et al. 2010). The results are in agreement with the commonly accepted view that serum level of transaminase returns to normal with the healing of hepatic parenchyma and the regeneration of hepatocytes (Fouraschen et al. 2013). Further, it is known that the stimulation of hepatic regeneration makes the liver more resistant against the damages by free radicals.
The glycogen deposits were depleted in AP. The findings document severe injury to the liver depending on the severity of inflammatory process in the pancreas (Andrzejewska et al. 1997). In the present study, CAR improves the hepatic glycogen content in treated AP rats. As a matter of fact, AP induced rats show an important histological sign by increasing fibrosis content around vessels. Oxidative stress may stimulate both the accumulation of collagen and extracellular matrix deposition (Kroy et al. 2014). Rappaport et al. (1983) reported that liver injuries induced changes in microcirculation that were associated with collagen deposition and consequently fibrosis. Acute pancreatitis is considered as high risk factor for vascular disorders (Björk and Arfors 1982). Hepatic microcirculation is through the sinusoids, and changes may contribute to hepatocellular dysfunction. The fibrosis underlying sinusoidal capillarization impede the rapid exchange of solutes between the sinusoidal space and hepatocytes, causing increased resistance to portal blood flow and portal hypertension (Bosch 2007).
On the other hand, the changes in the hepatic microcirculation have been suggested to be the result of systemic and regional hemodynamic changes, obstructions of the sinusoidal lumen and trapped blood cells (Ijaz et al. 2003). For this reason, hepatic congestion is a factor contributing to liver injury because of abnormal microcirculation in the liver (Masai et al. 2002; Nishi et al. 2007). Thus, the restoration of oxidative stress-induced vascular disorders in cerulein treated AP rats substantiates the protective action of drugs on liver. In this study, liver showed normal architecture. This finding shows that CAR did not induce any modification in the intracellular morphology of liver cell, which ultimately reveals its nontoxic nature at the given dosages. Moreover, CAR can save the tissue from congestion, fibrosis and lipid accumulation particular for AP-induced vascular disorders. In fact, CAR has long been used medicinally, proving to be beneficial in the treatment of circulatory problems and lymphatic system diseases (Esmaeili 2013). Our data reveal that the increasing dose of CAR displays a potent direct effect on liver cells in vivo and prevents leucocyte infiltration. Cerulein can trigger the accumulation of leucocytes and their adherence to the capillary wall. Therefore, it is likely that ROS play a central role in perpetuating the pancreatic inflammation and the development of extrapancreatic complication. The antioxidants are potentially useful in positively influencing the systemic redox balance and in consequence tissue inflammation and MDA (Turkez and Togar 2010; Fabian et al. 2013). This is in accordance with the results obtained by Silva et al. (2012), which demonstrated that the increasing dose of CAR was effective to protect against inflammation in gastric lesions. Additionally, CAR is able to control receptors that play an important role in inflammation (Hotta et al. 2010).
In conclusion, it is evident that increasing doses of CAR is capable of modulating the levels of LPO and significantly increases the endogenous antioxidant defense mechanisms in AP-induced hepatic damages by the present findings. Our results also show that the significant decrease in the levels of serum markers was prevented by CAR treatment. Such positive effects would be expected to improve the health. Then, we suggest that CAR has potential medicinal beneficial effects and supplementation of this plant material could be applied in the future to decrease detrimental effects of AP in liver.
References
- Abdel-Wahab B, Metwally M. Ginkgo biloba enhances the anticonvulsant and neuroprotective effects of sodium valproate against kainic acid-induced seizures in mice. J Pharmacol Toxicol. 2011;6:679–690. doi: 10.3923/jpt.2011.679.690. [DOI] [Google Scholar]
- Adorjan B, Buchbauer G. Biological properties of essential oils: an updated review. Flavour Fragr J. 2010;25:407–426. doi: 10.1002/ffj.2024. [DOI] [Google Scholar]
- Akkol EK, Orhan IE, Yeşilada E. Anticholinesterase and antioxidant effects of the ethanol extract, ethanol fractions and isolated flavonoids from Cistus laurifolius L. leaves. Food Chem. 2012;131:626–631. doi: 10.1016/j.foodchem.2011.09.041. [DOI] [Google Scholar]
- Andrzejewska A, Długosz J, Jurkowska G. The liver ultrastructure in caerulein and taurocholate acute pancreatitis in the rats. Rocz Akad Med Bialymst. 1997;43:117–136. [PubMed] [Google Scholar]
- Aristatile B, Al-Numair KS, Veeramani C, Pugalendi KV. Effect of carvacrol on hepatic marker enzymes and antioxidant status in d-galactosamine-induced hepatotoxicity in rats. Fundam Clin Pharmacol. 2009;23:757–765. doi: 10.1111/j.1472-8206.2009.00721.x. [DOI] [PubMed] [Google Scholar]
- Ayari S, Dussault D, Millette M, Hamdi M, Lacroix M. Response of Bacillus cereus to γ-irradiation in combination with carvacrol or mild heat treatment. J Agric Food Chem. 2010;58:8217–8224. doi: 10.1021/jf101044f. [DOI] [PubMed] [Google Scholar]
- Aydin E, Turkez H, Keles MS. The effect of carvacrol on healthy neurons and N2a cancer cells: some biochemical, anticancerogenicity and genotoxicity studies. Cytotechnology. 2014;66:149–157. doi: 10.1007/s10616-013-9547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beena Kumar D, Rawat DS. Synthesis and antioxidant activity of thymol and carvacrol based Schiff bases. Bioorg Med Chem Lett. 2013;23:641–645. doi: 10.1016/j.bmcl.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- Björk J, Arfors K-E. Oxygen free radicals and leukotriene B4 induced increase in vascular leakage is mediated by polymorphonuclear leukocytes. Agents Actions Suppl. 1982;11:63–72. [PubMed] [Google Scholar]
- Bosch J. Vascular deterioration in cirrhosis: the big picture. J Clin Gastroenterol. 2007;41:S247–S253. doi: 10.1097/MCG.0b013e3181572357. [DOI] [PubMed] [Google Scholar]
- Boskabady MH, Jalali S. Effect of carvacrol on tracheal responsiveness, inflammatory mediators, total and differential WBC count in blood of sensitized guinea pigs. Exp Biol Med. 2013;238:200–208. doi: 10.1177/1535370212474604. [DOI] [PubMed] [Google Scholar]
- Cacciatore I, Caccuri AM, Di Stefano A, Luisi G, Nalli M, Pinnen F, Ricci G, Sozio P. Synthesis and activity of novel glutathione analogues containing an urethane backbone linkage. Farmaco. 2003;58:787–793. doi: 10.1016/S0014-827X(03)00135-6. [DOI] [PubMed] [Google Scholar]
- Cacciatore I, Caccuri AM, Cocco A, De Maria F, Di Stefano A, Luisi G, Pinnen F, Ricci G, Sozio P, Turella P. Potent isozyme-selective inhibition of human glutathione s-transferase A1-1 by a novel glutathione S-conjugate. Amino Acids. 2005;29:255–261. doi: 10.1007/s00726-005-0232-7. [DOI] [PubMed] [Google Scholar]
- Cheshchevik VT, Lapshina EA, Dremza IK, Zabrodskaya SV, Reiter RJ, Prokopchik NI, Zavodnik IB. Rat liver mitochondrial damage under acute or chronic carbon tetrachloride-induced intoxication: protection by melatonin and cranberry flavonoids. Toxicol Appl Pharmacol. 2012;261:271–279. doi: 10.1016/j.taap.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Cingolani GM, Di Stefano A, Mosciatti B, Napolitani F, Giorgioni G, Ricciutelli M, Claudi F. Synthesis of L-(+)-3-(3-hydroxy-4-pivaloyloxybenzyl)-2,5-diketomorpholine as potential prodrug of L-dopa. Bioorg Med Chem Lett. 2000;10:1385–1388. doi: 10.1016/S0960-894X(00)00249-3. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- El-Demerdash F, Yousef M, El-Naga N. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Elhabazi K, Aboufatima R, Benharref A, Zyad A, Chait A, Dalal A. Study on the antinociceptive effects of Thymus broussonetii Boiss extracts in mice and rats. J Ethnopharmacol. 2006;107:406–411. doi: 10.1016/j.jep.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Esmaeili A. Biological activities and chemical composition of the stems and roots of Helichrysum oligocephalum DC grown in Iran. Pak J Pharm Sci. 2013;26:599–604. [PubMed] [Google Scholar]
- Fabian E, Pölöskey P, Kósa L, Elmadfa I, Réthy LA. Nutritional supplements and plasma antioxidants in childhood asthma. Wien Klin Wochenschr. 2013;125:309–315. doi: 10.1007/s00508-013-0359-6. [DOI] [PubMed] [Google Scholar]
- Fouraschen SM, de Ruiter PE, Kwekkeboom J, de Bruin RW, Kazemier G, Metselaar HJ, Tilanus HW, van der Laan LJ, de Jonge J. mTOR signaling in liver regeneration: rapamycin combined with growth factor treatment. World J Transpl. 2013;3:36. doi: 10.5500/wjt.v3.i3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyikoglu F, Turkez H. Protective effect of sodium selenite on genotoxicity to human whole blood cultures induced by aflatoxin B-1. Braz Arch Biol Technol. 2005;48:905–910. doi: 10.1590/S1516-89132005000800006. [DOI] [Google Scholar]
- Geyikoglu F, Turkez H. Boron compounds reduce vanadium tetraoxide genotoxicity in human lymphocytes. Environ Toxicol Pharmacol. 2008;26:342–347. doi: 10.1016/j.etap.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Geyikoglu F, Turkez H, Keles SM. The role of fruit juices in the prevention of aluminum sulphate toxicity in human blood in vitro. Fresenius Environ Bull. 2005;14:878–883. [Google Scholar]
- Giorda CB, Nada E, Tartaglino B, Marafetti L, Gnavi R. A systematic review of acute pancreatitis as an adverse event of type 2 diabetes drugs: from hard facts to a balanced position. Diabetes Obes Metab. 2014;16:1041–1047. doi: 10.1111/dom.12297. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Uchida T, Hasegawa A, Asai N, Noguchi T. Danaparoid sodium prevents cerulein-induced acute pancreatitis in rats. Shock. 2009;32:94–99. doi: 10.1097/SHK.0b013e31818ec2c2. [DOI] [PubMed] [Google Scholar]
- Hashemipour H, Kermanshahi H, Golian A, Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Heuking S, Iannitelli A, Di Stefano A, Borchard G. Toll-like receptor-2 agonist functionalized biopolymer for mucosal vaccination. Int J Pharm. 2009;381:97–105. doi: 10.1016/j.ijpharm.2009.03.039. [DOI] [PubMed] [Google Scholar]
- Hotta M, Nakata R, Katsukawa M, Hori K, Takahashi S, Inoue H. Carvacrol, a component of thyme oil, activates PPARα and γ and suppresses COX-2 expression. J Lipid Res. 2010;51:132–139. doi: 10.1194/jlr.M900255-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz S, Yang W, Winslet MC, Seifalian AM. Impairment of hepatic microcirculation in fatty liver. Microcirculation. 2003;10:447–456. doi: 10.1038/sj.mn.7800206. [DOI] [PubMed] [Google Scholar]
- Inkielewicz-Stepniak I, Santos-Martinez MJ, Medina C, Radomski MW. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int J Nanomed. 2014;9:1677–1687. doi: 10.2147/IJN.S59172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar S, Madankumar A, Asokkumar S, Raghunandhakumar S, Kamaraj S, Divya MGJ, Devaki T. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem. 2012;360:51–60. doi: 10.1007/s11010-011-1043-7. [DOI] [PubMed] [Google Scholar]
- Korkmaz T, Kahramansoy N, Kilicgun A, Firat T. The effect of erythropoietin to pulmonary injury and mast cells secondary to acute pancreatitis. BMC Res Notes. 2014;7:267. doi: 10.1186/1756-0500-7-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroy DC, Schumacher F, Ramadori P, Hatting M, Bergheim I, Gassler N, Boekschoten MV, Müller M, Streetz KL, Trautwein C. Hepatocyte specific deletion of c-Met leads to the development of severe non-alcoholic-steatohepatitis in mice. J Hepatol. 2014;61:883–890. doi: 10.1016/j.jhep.2014.05.019. [DOI] [PubMed] [Google Scholar]
- LaRusch J, Solomon S, Whitcomb DC. Pancreatitis overview. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews®. Seattle: University of Washington; 2014. [Google Scholar]
- Lee JH, An CS, Yun BS, Kang KS, Lee YA, Won SM, Gwag BJ, Cho SI, Hahm KB. Prevention effects of ND-07, a novel drug candidate with a potent antioxidative action and anti-inflammatory action, in animal models of severe acute pancreatitis. Eur J Pharmacol. 2012;687:28–38. doi: 10.1016/j.ejphar.2012.04.048. [DOI] [PubMed] [Google Scholar]
- Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal. 2009;11:135–165. doi: 10.1089/ars.2008.2109. [DOI] [PubMed] [Google Scholar]
- Liang ZH, Qin MB, Tang GD, Yang HY, Su J, Huang JA. Melatonin reduces inflammation and recovers endogenous ghrelin in acute necrotizing pancreatitis in rats. Mol Med Rep. 2014;9:2599–2605. doi: 10.3892/mmr.2014.2132. [DOI] [PubMed] [Google Scholar]
- Masai T, Sawa Y, Ohtake S, Nishida T, Nishimura M, Fukushima N, Yamaguchi T, Matsuda H. Hepatic dysfunction after left ventricular mechanical assist in patients with end-stage heart failure: role of inflammatory response and hepatic microcirculation. Ann Thorac Surg. 2002;73:549–555. doi: 10.1016/S0003-4975(01)03510-X. [DOI] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Müller S, Kaiser H, Krüger B, Fitzner B, Lange F, Bock CN, Nizze H, Ibrahim SM, Fuellen G, Wolkenhauer O, Jaster R. Age-dependent effects of UCP2 deficiency on experimental acute pancreatitis in mice. PLoS ONE. 2014;9:e94494. doi: 10.1371/journal.pone.0094494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. Washington: National Academy Press; 1996. [Google Scholar]
- Nepomnyashchikh LM, Bakarev MA, Vasilyev AV, Protsenko SI. Pathomorphological analysis of the pancreaticoduodenal organs in experimental pancreonecrosis induced by trypsin injection. Bull Exp Biol Med. 2013;155:249–254. doi: 10.1007/s10517-013-2125-1. [DOI] [PubMed] [Google Scholar]
- Nishi H, Takahashi T, Matsumiya G, Takano H, Ichikawa H, Miyagawa S, Sawa Y. Preoperative assessment of congestive liver dysfunction using technetium-99 m galactosyl human serum albumin liver scintigraphy in patients with severe valvular heart disease. Surg Today. 2007;37:564–569. doi: 10.1007/s00595-006-3460-x. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Oliveira NM, Ferreira FA, Yonamine RY, Chehter EZ. Antiretroviral drugs and acute pancreatitis in HIV/AIDS patients: is there any association? A literature review. Einstein (Sao Paulo, Brazil) 2014;12:112–119. doi: 10.1590/S1679-45082014RW2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gutierrez RM, Damian-Guzman M. Meliacinolin: a potent α-glucosidase and alpha-amylase inhibitor isolated from Azadirachta indica leaves and in vivo antidiabetic property in streptozotocin-nicotinamide-induced type 2 diabetes in mice. Biol Pharm Bull. 2012;35:1516–1524. doi: 10.1248/bpb.b12-00246. [DOI] [PubMed] [Google Scholar]
- Rappaport A, MacPhee P, Fisher M, Phillips M. The scarring of the liver acini (cirrhosis) Virchows Arch A. 1983;402:107–137. doi: 10.1007/BF00695054. [DOI] [PubMed] [Google Scholar]
- Rispoli V, Rotiroti D, Carelli V, Liberatore F, Scipione L, Marra R, Giorgioni G, Di Stefano A. Choline pivaloyl esters improve in rats cognitive and memory performances impaired by scopolamine treatment or lesions of the nucleus basalis of Meynert. Neurosci Lett. 2004;356:199–202. doi: 10.1016/j.neulet.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Rocha-Guzmán NE, Gallegos-Infante JA, González-Laredo RF, Ramos-Gómez M, Rodriguez-Munoz ME, Reynoso-Camacho R, Rocha-Uribe A, Roque-Rosales MR. Antioxidant effect of oregano essential oil and mother liquors. Food Chem. 2007;102:330–335. doi: 10.1016/j.foodchem.2006.05.024. [DOI] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Saei-Dehkordi SS, Tajik H, Moradi M, Khalighi-Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol. 2010;48:1562–1567. doi: 10.1016/j.fct.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Sawa T, Ohshima H. Nitrative DNA damage in inflammation and its possible role in carcinogenesis nitric oxide. Biol Chem. 2006;14:91–100. doi: 10.1016/j.niox.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Shiba H, Zhu X, Arakawa Y, Irefin S, Wang B, Trenti L, Fung JJ, Kelly DM. Oxygen consumption predicts outcome in porcine partial liver grafts. J Surg Res. 2014;189:335–339. doi: 10.1016/j.jss.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Silva FV, Guimarães AG, Silva ER, Sousa-Neto BP, Machado FD, Quintans-Júnior LJ, Arcanjo DD, Oliveira FA, Oliveira RC. Anti-inflammatory and anti-ulcer activities of carvacrol, a monoterpene present in the essential oil of oregano. J Med Food. 2012;15:984–991. doi: 10.1089/jmf.2012.0102. [DOI] [PubMed] [Google Scholar]
- Tasic T, Grgov S, Nagorni A, Benedeto-Stojanov D. Comparison of biohumoral and morphological parameters in acute pancreatitis. Srp Arh Celok Lek. 2014;142:29–33. doi: 10.2298/SARH1402029T. [DOI] [PubMed] [Google Scholar]
- Teixeira RB, Kelley J, Alpert H, Pardo V, Vaamonde CA. Complete protection from gentamicin-induced acute renal failure in the diabetes mellitus rat. Kidney Int. 1982;21:600–612. doi: 10.1038/ki.1982.67. [DOI] [PubMed] [Google Scholar]
- Turkez H, Togar B. The genotoxic and oxidative damage potential of olanzapine in vitro. Toxicol Ind Health. 2010;26:583–588. doi: 10.1177/0748233710373090. [DOI] [PubMed] [Google Scholar]
- Turkez H, Tatar A, Hacimuftuoglu A. Boric acid as a protector against paclitaxel genotoxicity. Acta Biochim Pol. 2010;57:95–97. [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Mokhtar YI. Eicosapentaenoic acid protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced hepatic toxicity in cultured rat hepatocytes. Cytotechnology. 2012;64:15–25. doi: 10.1007/s10616-011-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Yousef MI, Celik K, Bakir TO. Ameliorative effect of supplementation with L: -glutamine on oxidative stress, DNA damage, cell viability and hepatotoxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat hepatocyte cultures. Cytotechnology. 2012;64:687–699. doi: 10.1007/s10616-012-9449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkvatan A, Erden A, Turkoglu MA, Secil M, Yuce G. Imaging of acute pancreatitis and its complications. Part 2: complications of acute pancreatitis. Diagn Interv Imaging. 2014;96:161–169. doi: 10.1016/j.diii.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Undeger U, Basaran A, Degen GH, Basaran N. Antioxidant activities of major thyme ingredients and lack of (oxidative) DNA damage in V79 Chinese hamster lung fibroblast cells at low levels of carvacrol and thymol. Food Chem Toxicol. 2009;47:2037–2043. doi: 10.1016/j.fct.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Yang CJ, Chen J, Phillips AR, Windsor JA, Petrov MS. Predictors of severe and critical acute pancreatitis: a systematic review. Dig Liver Dis. 2014;46:446–451. doi: 10.1016/j.dld.2014.01.158. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Deans SG. Effect of thyme oil and thymol dietary supplementation on the antioxidant status and fatty acid composition of the ageing rat brain. Br J Nutr. 2000;83:87–93. [PubMed] [Google Scholar]
- Yu H, Zhang ZL, Chen J, Pei A, Hua F, Qian X, He J, Liu CF, Xu X. Carvacrol, a food-additive, provides neuroprotection on focal cerebral ischemia/reperfusion injury in mice. PLoS ONE. 2012;7:e33584. doi: 10.1371/journal.pone.0033584. [DOI] [PMC free article] [PubMed] [Google Scholar]