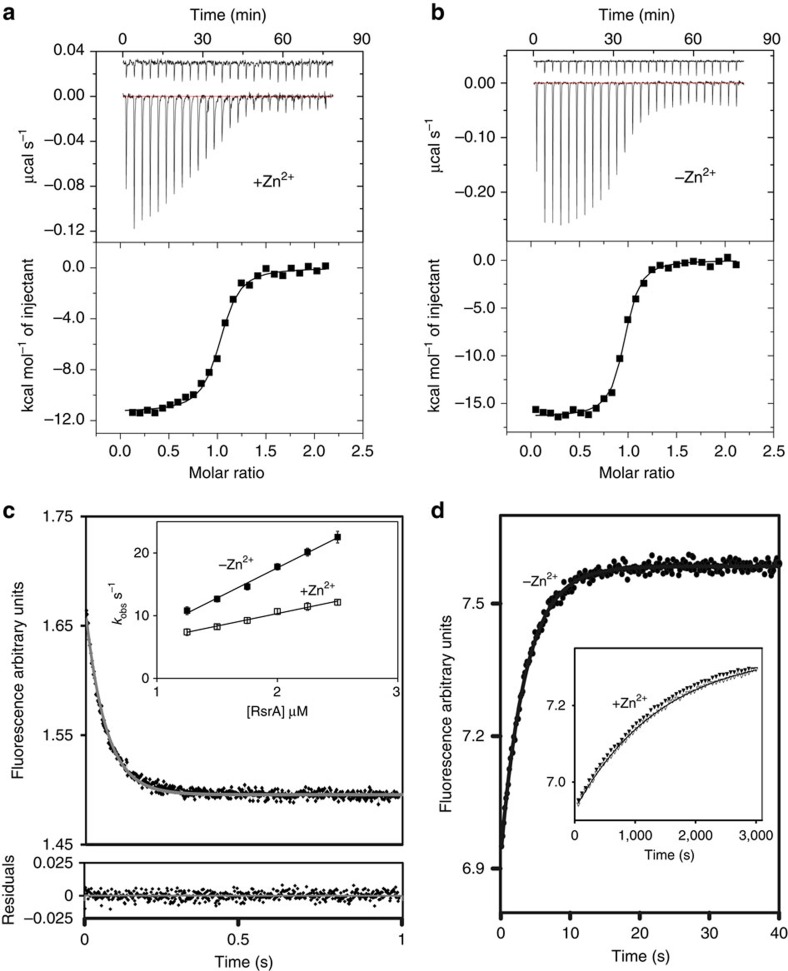
Figure 3. Zinc slows the dissociation rate of the high-affinity RsrAred.Zn2+-σR complex.
Conditions used for all experiments were 50 mM Tris (pH 7.5) buffer containing 100 mM NaCl and 2 mM DTT. Temperature was either 35 °C (a,b) or 25 °C (c,d). (a) Competition ITC data for RsrAred.Zn2+ (100 μM) binding σR (10 μM, cell concentration) in the presence of RsrA* Cys11Ser Cys44Ser (50 μM). RsrA* Cys11Ser Cys44Ser binds σR with a weaker affinity than wild-type RsrA (Supplementary Table 1). Control data show titration of RsrAred.Zn2+ into buffer containing 50 μM RsrA* Cys11Ser Cys44Ser. Fitted parameters for a competitive binding site model from three independent measurements were N=1.01±0.05, Kd=0.78±0.034 nM, ΔH=−23.05±1.10 kcal mol−1, ΔS=−38.70±3.10 cal mol−1 deg−1. (b) Direct ITC data for RsrAred (100 μM) binding σR (10 μM) in the absence of zinc. Control data show titration of RsrAred into buffer. Fitted parameters for a single site-binding model were N=0.97±0.004, Kd=79.3±4.2 nM, ΔH=−16.26±0.13 kcal mol−1, ΔS=−20.3±4.34 cal mol−1 deg−1. (c) Main panel: tryptophan emission fluorescence stopped-flow association data for RsrAred.Zn2+ binding σR under pseudo-first-order conditions (λex, 295 nm). Residuals to the fit of a single exponential are shown below the panel. Inset: pseudo-first-order plot of observed rates (kobs) as a function of RsrA concentration in the presence and absence of stoichiometric zinc (with associated error bars). See Supplementary Table 2 for derived values of kon. Error bars are within the data point symbols. (d) Dissociation of the RsrAred–σR complex measured by competition stopped-flow in which a 10-fold excess of σR Trp88Ile Trp119Ile was used to displace wild-type σR (Methods). Main panel shows data for the RsrAred–σR complex in the absence of bound zinc. Inset: dissociation data for the RsrAred–σR complex in the presence of 1 and 3 equiv. of zinc (open and filled triangles, respectively). See Supplementary Table 2 for values of koff.