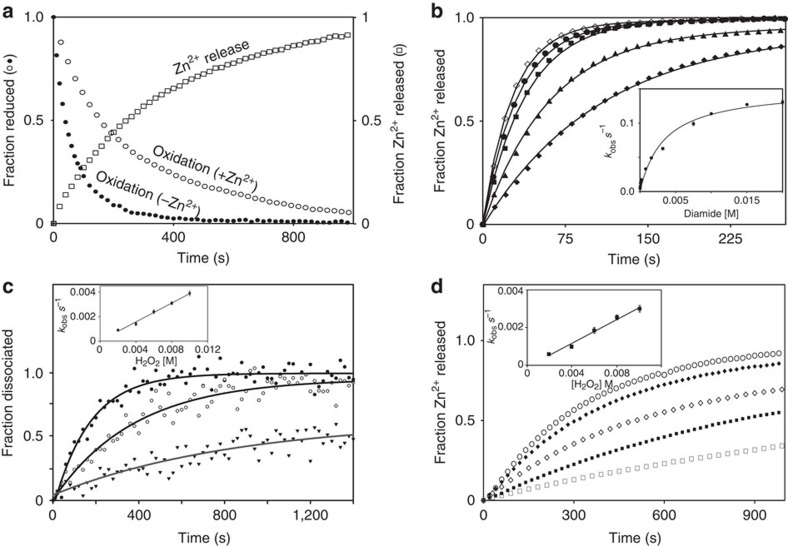
Figure 4. Zinc release is the rate-limiting step in RsrA oxidation.
Experiments were conducted at 25 °C in 50 mM Tris (pH 7.5) buffer containing 100 mM NaCl. (a) Oxidation of the RsrA–σR complex on treatment with diamide under second-order conditions (25 μM). The fraction of reduced RsrA was determined by the change in diamide absorbance at 320 nm (Supplementary Fig. 2a) in the presence and absence of bound zinc. Zinc release was monitored at 500 nm using the PAR assay. The two methods showed good agreement for the bimolecular rate constant for diamide-induced oxidation of RsrAred.Zn2+ (183±6 and 195±11 M−1 s−1, respectively). (b) Zinc release from the RsrAred.Zn2+–σR complex (2 μM) on treatment with increasing concentrations of diamide under pseudo-first-order conditions monitored by the PAR assay; 25 μM (filled diamonds), 50, 100, 150 and 200 μM (open diamonds). Inset shows variation of kobs with diamide concentration (with associated error bars). Data were fitted to the Michealis–Menten equation, with fitted parameters of K1=0.7 mM and k2=0.15 s−1 (Supplementary Fig. 2b). The corresponding bimolecular rate constant (k2/K1=214 M−1 s−1) is in reasonable agreement with values obtained in a. (c) Oxidation-induced dissociation of the RsrAred.Zn2+–σR complex (2 μM) monitored by tryptophan fluorescence on treatment with H2O2 under pseudo-first-order conditions. Three H2O2 concentrations are shown as follows: 1 mM (triangles), 2 mM (open circles) and 6 mM (closed circles). Inset, pseudo-first-order plot (with associated error bars) from which the bimolecular rate constant for H2O2-induced dissociation of the complex was obtained (0.39±0.08 M−1 s−1). (d) Zinc release from the RsrAred.Zn2+–σR complex (2 μM) on treatment with increasing concentrations of H2O2 (2–10 mM) under pseudo-first-order conditions. Inset: pseudo-first-order plot (with associated error bars) from which the bimolecular rate constant for the H2O2-induced zinc dissociation was obtained (0.32±0.06 M−1 s−1).