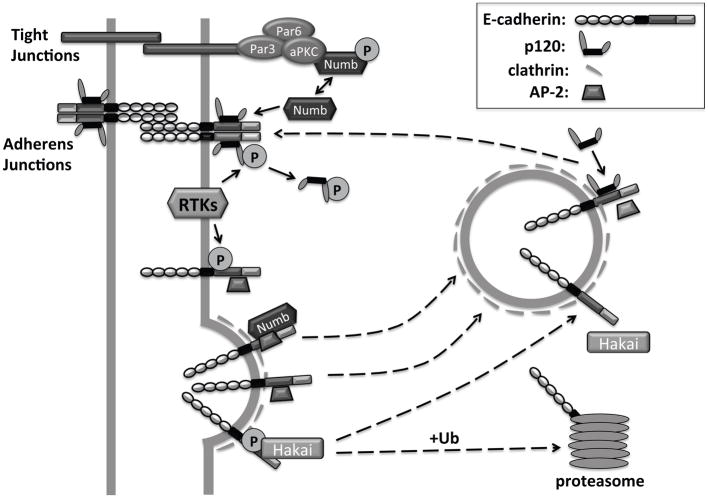
Figure 1. Cadherin stabilization by p120 catenin.
p120 binding to the cadherin juxtamembrane domain stabilizes cadherins at the cell membrane and promotes the formation of adherens junctions. Removal of p120 from the cadherin complex can proceed via multiple mechanisms, including phosphorylation of p120 and/or E-cadherin downstream of receptor tyrosine kinases (RTKs), or association with non-phosphorylated Numb, a protein normally phosphorylated by aPKC. p120 dissociation uncovers an adaptor protein 2 (AP-2) binding motif, as well as a phosphorylation-dependent motif for the recruitment of the E3 ligase Hakai. AP-2 binding promotes clathrin-dependent endocytosis of E-cadherin. The endocytosed E-cadherin can be recycled back to the membrane, upon binding to p120. Alternatively, Hakai-induced ubiquitination of E-cadherin can lead to cadherin degradation in the proteasome.