ABSTRACT
The zebrafish (Danio rerio) has become a popular model for human cardiac diseases and pharmacology including cardiac arrhythmias and its electrophysiological basis. Notably, the phenotype of zebrafish cardiac action potential is similar to the human cardiac action potential in that both have a long plateau phase. Also the major inward and outward current systems are qualitatively similar in zebrafish and human hearts. However, there are also significant differences in ionic current composition between human and zebrafish hearts, and the molecular basis and pharmacological properties of human and zebrafish cardiac ionic currents differ in several ways. Cardiac ionic currents may be produced by non-orthologous genes in zebrafish and humans, and paralogous gene products of some ion channels are expressed in the zebrafish heart. More research on molecular basis of cardiac ion channels, and regulation and drug sensitivity of the cardiac ionic currents are needed to enable rational use of the zebrafish heart as an electrophysiological model for the human heart.
KEYWORDS: calcium channels, cardiac action potential, cardiac ionic currents, potassium channels, sodium channels
Introduction
Zebrafish, a tropical teleost fish species is increasingly used as a model for human cardiac electrophysiology, arrhythmias and drug screening.6,12,53,56 The use of zebrafish models is based, besides the several well established technical advantages (optical transparency, large offspring number, rapid development, genetic amenability), on the promise that genetic and molecular mechanisms behind cardiac physiologies are conserved throughout the vertebrate evolution from fishes to humans.32 Indeed, about 71% of human genes have at least one ortholog in the zebrafish genome.23 On the other hand evolution has resulted in enormous diversity of life-forms in fishes via genetic adaptation to different habitats. This diversity is largely based on the whole genome duplication early in the teleost lineage and subsequent subfunctionalization, neofunctionalization and loss of genes, and reorganization of gene regulation.57 As a result, over 3100 human genes have at least 2 orthologues in the zebrafish genome and both species have several thousand unique genes without an ortholog in each other's genome.23 Therefore, design of experiments and interpretation of the results cannot be based on the assumption that molecular entities and regulatory pathways behind the functions under study are always the same in zebrafish and humans. In order to draw correct conclusions from the zebrafish model to human cardiac electrophysiology, ion channel functions that are shared must be discriminated from those that are derived and novel in zebrafish and humans.7 The recent analysis of the zebrafish Kir2 channel composition provides a striking example how different the molecular basis of cardiac ionic current (IK1) can be in zebrafish and human hearts.17 This finding warrants a careful examination of other cardiac ionic currents as well.44 This overview examines the current state of knowledge on the zebrafish cardiac ion channels with relation to the human cardiac ion channels.
Action potentials
Action potential (AP) of the zebrafish ventricle shares the main characteristics of the human cardiac AP. Most importantly the zebrafish ventricular AP has a long plateau phase, similar to the human cardiac AP, and consequently a distinct QT-interval in the electrocardiogram (Figs. 1 and 2).29,63 All phases (0-4) of the cardiac AP, with the exception of the rapid phase-1 repolarization, are present in the zebrafish heart (Fig. 1).40 The absence of the phase-1 repolarization suggests that the transient outward current (ITo) is tiny or absent in the zebrafish heart.2 Furthermore, it should be noted, that as an ectotherm the duration of cardiac AP in zebrafish varies with body temperature within its thermal tolerance range between 6.2°C and 41.7°C.34 In comparison to the hearts of endothermic vertebrates, ion channel function in ectothermic fishes is scaled down to lower temperatures. The duration of zebrafish ventricular AP at 19°C is similar to the human ventricular AP at 37°C, while at the human body temperature the duration of the zebrafish ventricular AP is only about one fifth of the duration of the human cardiac AP (Fig. 1).
Figure 1.
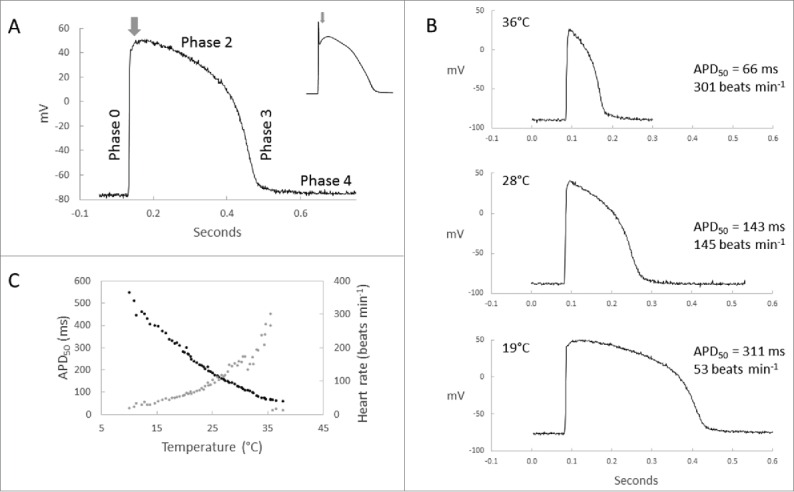
Ventricular action potential of the zebrafish heart. (A) Typical action potential of the zebrafish ventricle shows fast upstroke (phase-0), long plateau (phase-2), rapid repolarization (phase-3) and stable resting membrane potential (phase-4), but lacks the fast phase-1 repolarization (arrow), which is typical for the human cardiac action potential (top right). As an ectotherm action potential duration and heart rate in the zebrafish is temperature-dependent within the thermal-tolerance range of the species. (B) Representative microelectrode recordings of ventricular AP at selected temperatures (36°C, 28°C and 19°C) and (C) within the whole range of temperatures between 10°C and 36°C.
Figure 2.
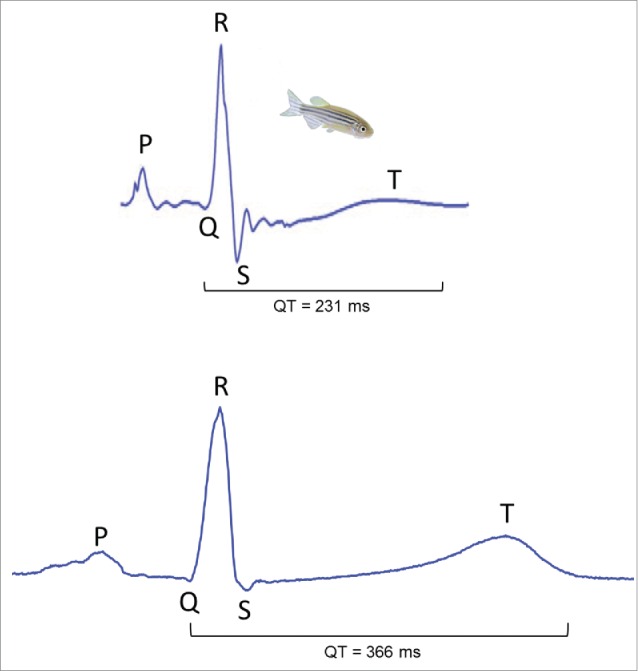
Comparison of human and zebrafish electrocardiograms (ECG). (Top) ECG of an adult zebrafish at 23°C (kindly provided by prof. Tzung Hsiai). (Bottom) ECG of a healthy 43-year old human male. For direct comparison of zebrafish and human ECGs both recordings are shown in the same time scale. Similar to the human electrocardiogram, P, QRS and T waves are clearly distinguishable in the zebrafish ECG.
Inward rectifier K+ current (IK1)
The cardiac inwardly rectifying K+ current (IK1) stabilizes the resting membrane potential (phase-4) and is responsible for shaping the initial depolarization and the final phase-3 repolarization of the cardiac AP.22 A robust background IK1 is present in atrial and ventricular myocytes of the adult zebrafish, and similar to mammalian hearts, the density of IK1 is markedly higher in ventricular than atrial myocytes.40 There is, however, remarkable differences in cardiac IK1 channel composition between human and zebrafish hearts. The human cardiac IK1 is produced by Kir2.1, Kir2.2 and Kir2.3 channels. Kir2.1 channel forms about 50% of the Kir2 transcripts in the right ventricle,14 while in the right atrium Kir2.3 is the main Kir2 isoform (56%) with less contributions by Kir2.2 (31%) and Kir2.1 (13%) channels.14 In the zebrafish heart 6 Kir2 channel isoforms are expressed at the transcript level. In the ventricle an ortholog to the mammalian Kir2.4 channel is the main Kir2 channel isoform constituting 92.9% of the total Kir2 population. In the zebrafish atrium, Kir2.2a and Kir2.4 form 64.7% and 29.3% of the Kir2 transcripts, respectively.17 Notably Kir2.1a, Kir2.1b and Kir2.3 together form less than 6% and 1% of the Kir2 transcripts in zebrafish atrium and ventricle, respectively. Thus, the Kir2 composition of the zebrafish heart is dominated by an isoform, which in mammals is hardly expressed in cardiac myocytes.13,24,54
There are also functional differences between zebrafish and mammalian Kir2 channels. The zebrafish Kir2.4 is about 2 orders of magnitude more sensitive to Ba2+ block than its mammalian counterpart. On the other hand zebrafish Kir2.1a is almost an order of magnitude less sensitive to Ba2+ block than the mammalian Kir2.1 channels.17
Delayed rectifier K+ currents (IKr, IKs)
The two major repolarizing K+ currents, that end the long plateau phase of the human cardiac AP, are IKr and IKs. They are active during the phases 2 and 3 of the cardiac AP. IKr is the main repolarizing current of the human heart, while IKs comes into a play when more repolarizing reserves are needed, e.g. under β-adrenergic activation and with increasing heart rates.50
In the human heart the pore forming component of the IKr channel (human erg1 or herg) is encoded by the KCNH2 gene 49,59 and the functional IKr channels are heterotetramers of the 2 splice variants of the KCNH2 gene, herg1a and herg1b.26 IKr is also the main repolarizing K+ current in atrial and ventricular myocytes of the zebrafish heart.40,60 Interestingly, in the zebrafish heart IKr is not produced by erg1 channels but by erg2 channels, which are encoded by the zebrafish ortholog to the mammalian KCNH6 gene.30 In the zebrafish heart 4 erg genes are expressed (KCNH6, KCNH2A, KCNH2B and KCNH7), KCNH6 being clearly the dominant isoform (99.1 ± 0.15 and 99.4 ± 0.08% of the transcripts in atrium and ventricle, respectively) (Fig. 3). In mammals erg2 is expressed in the nervous tissue but not in the heart, while in zebrafish erg1 is very weakly expressed in the heart.30 Thus, the IKr is generated by non-orthologous genes in human (erg1) and zebrafish (erg2) hearts.
Figure 3.
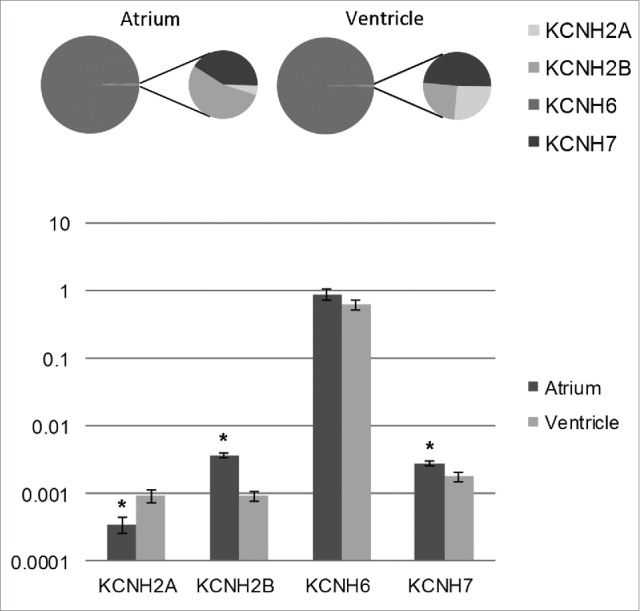
Transcript expression of the 4 Kv11 (erg) channel family members in the zebrafish heart. The bar graph shows abundances of KCNH transcripts in atrial and ventricular muscle of the zebrafish normalized to the expression of the housekeeping gene DnaJA2 (mean ± SEM; n = 6). The pie charts indicate relative proportions (%) of each KCNH transcript in the whole KCNH pool in atrium and ventricle. Statistically significant differences (Student's t-test; p < 0.05) between atrium and ventricle are indicated with an asterisk.
Biophysical properties of the human erg1 and the zebrafish erg2 also differ. Steady-state activation and inactivation of the zebrafish IKr are −15 mV and +23 mV, respectively, different from the respective values of the human IKr, when measured in the same cellular environment (Xenopus oocytes). Furthermore, the presence of herg1b splice variant in the channel assembly affects drug sensitivity of the human IKr.1,27,38 Mutations in the zebrafish erg channel cause similar electrophysiological features that are typical for short QT and long QT syndromes in humans.3,16 QT-prolonging drugs induce AP prolongation, bradycardia and atrioventricular conduction block in zebrafish embryos.28,39
Different from the human heart, there is no direct demonstration on the presence of IKs in the zebrafish cardiac myocytes.2,40 The mammalian cardiac IKs is generated by a heteromultimeric assembly of Kv7.1 (KCNQ1 gene) α-subunits and minK (KCNE1 gene) β-subunits. Although IKs has not been reported for zebrafish cardiac myocytes,2,40 transcripts of the zebrafish orthologues to the mammalian KCNQ1 and KCNE1 genes are expressed in the zebrafish heart.62 Experiments with another fish species (Carassius carassius) of the zebrafish family (Cyprinidae) suggest that, when present, the fish cardiac IKs is mainly produced by homotetramers of Kv7.1 channel without the MinK β-subunit and with distinctly different electrophysiological properties.20 Unlike the mammalian IKs, the fish cardiac IKs has fast activation kinetics, is independent of stimulation frequency, is not augmented by cAMP-dependent pathway and is less sensitive to chromanol 239B block than its mammalian counterpart.20 Similar to the human heart also other members of the Kv7 channel subfamily (KCNQ2-4) are expressed to some extent in the zebrafish heart.35,62
Calcium currents
Ca2+ currents (ICa) maintain the long plateau phase of cardiac APs (phase-2) and provide activator Ca2+ for contraction. Atrial and ventricular myocytes of the zebrafish heart have both T-type (ICaT) and L-type (ICaL) Ca2+ currents,5,8,40,47 while in adult mammalian heart the presence of ICaT is mainly restricted to the sinoatrial node and the conductive pathways. Notably, the density of ICa,L is markedly larger in zebrafish ventricular myocytes than in human cardiomyocytes.65
In mammals, there are 4 alpha1 subunits of the L-type Ca2+ channels (α1S, α1C, α1D, α1F, or Cav1.1-4) Cav1.2 being the dominant cardiac isoform.37 In the zebrafish heart ventricular contraction is abolished by mutation in the α-1C subunit (Cav1.2) strongly suggesting that ICaL is produced by orthologous genes in humans and zebrafish. In the heart of adult zebrafish transcripts of the α-1D (Cav1.3) are also expressed.52 ICaT is an important component of pacemaker and conductive tissues, but usually absent in atrial and ventricular muscle of adult mammals. In this respect zebrafish is clearly different from humans and other mammals. Two α subunits of T-type Ca2+ channels (Cav3.1 and Cav3.2) are expressed in mammalian hearts.11,43 T-type channel composition of the zebrafish has not been studied, but Ni2+ sensitivity suggests that it might be an ortholog to the mammalian Cav3.1,40 while immunofluorescence findings suggest the presence of Cav3.2.2 The diversity of Ca2+ channel α-subunits is, however, much higher in fishes than mammals due to the whole genome duplication in the teleost lineage. Indeed in fugu (Fugu rubribes) 21 Ca2+ channel α-subunits have been found and as many as 16 of them are expressed in the heart.61
Sodium currents
The voltage-gated Na+ channels have a central role in excitability of myocardial cells, because they generate the rapid upstroke of the myocardial AP (phase-0) and determine the velocity of impulse transmission over the heart.15,46 A fast Na+ current (INa) exists in atrial and ventricular myocytes of the zebrafish heart.40,60 The rate of AP upstroke is slower in zebrafish atrial and ventricular muscle in comparison o human heart suggesting that INa density is significantly lower in the zebrafish heart.40 In cultured cardiac myocytes of the zebrafish embryos, INa current density is equal in atrial and ventricular myocytes, but the atrial INa activates at slightly more negative voltages in comparison to the ventricular INa.60
In larval cardiac tissue (72 hpf) of the zebrafish 2 α-subunits of Na+ channels (SCN5Laa and SCN5Lab), orthologous to the human cardiac SCN5A, are expressed.42 However, in the adult zebrafish heart only scn5Lab has been found.9 In contrast to the mammalian cardiac Na+ channel (SCN5A) the Na+ channels of the fish heart are about 3 orders of magnitude more sensitive to tetrodotoxin (TTX).21 This is due to the replacement of non-aromatic cysteine (C401) in the poor loop of the domain-I with an aromatic tyrosine (Y401) in the fish cardiac Na+ channels. Since this replacement is also present in both paralogs of the zebrafish cardiac Na+ channel, the zebrafish INa is also highly sensitive to TTX.9,40,58 Association of the zebrafish SCN5Lab with its beta1-subunit increases INa amplitude when expressed in the CHO cell line.10 Collectively these findings suggest that the paralogs of the zebrafish cardiac Na+ channels are orthologous to the main human cardiac isoform, but different from the mammalian INa the zebrafish cardiac INa is TTX-sensitive.
Other ionic currents
Practically nothing is known about the ligand-gated inward rectifiers currents, the ATP-sensitive K+ current (IKATP) and the acetylcholine-activated K+ current (IKACh) of the zebrafish heart. IATP has been recorded from cardiomyocytes of some fish species and therefore it is likely that similar current exists also in zebrafish cardiac myocytes. Three ATP-sensitive channels have been found in the zebrafish genome (Kir6.1, Kir6.2 and Kir6.3) located in different chromosomes.64 Synteny data suggests, however, that the Kir6.3 is in fact a paralogue of the Kir6.2 (i.e. Kir6.2b) (Table 1). In mammals the conductance pore of ATP-sensitive channels is produced by Kir6.1 and Kir6.2 channels, which assemble with sulfonylurea receptors to produce fully functional channels. No data is available on the expression of these channels in the zebrafish heart. In the goldfish (Carassius auratus) heart Kir6.1and Kir6.2 channels are expressed.
Table 1.
The current state of knowledge about the ionic currents and ion channels of the zebrafish heart.
| Current | Protein (α-subunit) | Gene | References |
|---|---|---|---|
| INa | Nav1.5a, Nav1.5b | SCN5LAa, SCN5LAb | 10,60 |
| ICaL | Cav1.2 Cav1.3a | CACNA1C CACNA1D | 8,40,47,52,65 |
| ICaT | Cav3.1 Cav3.2 | CACNA1G CACNA1H | 5,40 |
| IKr | Kv11.2 (Erg2) Kv11.1 (Erg1) Kv11.1 (Erg1) Kv11.3 (Erg3) | KCNH6 KCNH2A KCNH2B KCNH7 | 3,5,19,28,51 |
| IKs* | Kv7.1 | KCNQ1 | 62 |
| IK1 | Kir2.4 Kir2.2a Kir2.2b Kir2.1a Kir2.1b Kir2.3 | KCNJ14 KCNJ12A KCNJ12B KCNJ2A KCNJ2B KCNJ4 | 17,40 |
| IKACh IKATP** If | NK Kir6.1 Kir6.2A Kir6.2B HCN4 | NK KCNJ8 KCNJ11A KCNJ11B HCN4 | 5.40,60,64 |
The main ion channel isoforms (if known) are indicated in bold.
IKs current has not been found in the zebrafish heart;
IKATP current has not been measured in the zebrafish heart; NK, not known
Acetylcholine induces a large inwardly rectifying K+ current in atrial but not in ventricular myocytes of the zebrafish heart.40 However, the molecular basis of this current has not yet been resolved.
Pacemaker current (If or Ih) has been demonstrated to be present in cultured cardiac myocytes of embryonic zebrafish as well as in cultured atrial and ventricular myocytes of the adult zebrafish. Down-regulation of the Ih by a recessive slo mo mutation (which probably encodes a mitochondrial protein) causes depression of intrinsic heart rate, especially in the embryonic heart.60 The zebrafish Ih consist of slow and fast components suggesting that it is produced by 2 different channels similar to the human heart. Pacemaker tissue of the zebrafish heart demonstrates the presence of HCN4 channels as one of the likely candidates for coding the Ih channels.55
Conclusions and implications
Considering the increasing use of zebrafish as a model for human cardiac electrophysiology, cardiac arrhythmias and drug screening, our knowledge on the zebrafish cardiac ion channels, their biophysical properties, drug sensitivity, molecular basis and regulatory networks is still limited. The current knowledge indicates that human and zebrafish myocytes share several electrical properties, but on the other hand several differences also exist between human and zebrafish cardiac APs, ion channel function and ion channel molecular composition (Table 2). Zebrafish and human hearts both have a distinct plateau phase. In this respect the zebrafish heart is clearly a better model than the murine heart, which is characterized by a short AP plateau and strong reliance on ITo in AP repolarization.25,41 The ionic current basis of human and zebrafish cardiac APs is similar in that IK1 and IKr are the major repolarizing currents in both hearts. On the other hand, zebrafish ventricular AP, unlike human cardiac AP, does not have clear phase-1 repolarization suggesting that the transient outward current (ITo) is not expressed in the zebrafish heart.2 Unlike human heart, atrial and ventricular myocytes of the zebrafish heart have both ICaT and ICaL. IK1 and IKr are generated by different gene products in humans and zebrafish, and IKs may be absent from the zebrafish heart.
Table 2.
Similarities and differences of the zebrafish cardiac electrophysiology with the human cardiac electrophysiology.
| Similarities with human cardiac electrophysiology | Differences to human cardiac electrophysiology |
|---|---|
| Cardiac AP has a clear plateau phase. | Absence of the phase 1 repolarization (ITo not expressed) |
| A distinct QT interval in ECG | The slow component of the delayed rectifier IKs is not expressed in the zebrafish heart. |
| Similar fundamental current systems (INa, ICaL, IK) are present in atrial and ventricular myocytes. | Large ICaT is present in zebrafish atrial and ventricular myocytes. |
| IKr and IK1 are the main repolarizing currents. | IK1 is mainly generated by Kir2.4 and Kir2.2a channels. |
| IKr is mainly generated by erg2 (KCNH6) channels. | |
| The balance of inward currents: density of INa low and density of ICa high in comparison to the human heart |
A number of excellent techniques have been developed for recording electrical and mechanical activity of the zebrafish heart in vivo and in vitro, thereby making this small fish amenable for cardiac studies.4,31,33,63 Effects of disease causing mutations and pharmaceutical drugs on electrical excitation, rhythm and contraction of the zebrafish heart can be traced with high accuracy, making it putatively an interesting model for human cardiac electrophysiology. Due to the presence of ion channel paralogues in the zebrafish genome, differences in ion channel proteins and subunit composition of ion channels between zebrafish and human hearts, zebrafish is unlikely to be a good general-purpose model for the human cardiac electrophysiology. However, similarity of the major repolarizing (IK1, IKr) and depolarizing (INa, ICaL) currents, despite of their partially different molecular basis, makes the zebrafish heart an appropriate model for more specific issues of human cardiac excitation. Because various Kir2 channels and different erg channels are mutually similar in function, i.e., all Kir2 channels are strong inward rectifiers and all erg channels show fast inactivation, fast recovery from inactivation and slow deactivation,36 species-specific differences in channel compositions may not be critical, when studying disease causing mutations or using functional knock-downs and knock-outs. The same applies to the inward currents, INa and ICaL. However, a prerequisite for these studies is that the ion channel composition of the zebrafish heart under study is known and correct channel paralogs and isoforms are targeted.
The special characteristics of the zebrafish cardiac ionic currents are more problematic in drug screening since different ionic currents, even if qualitatively similar to human counterparts, may show significant quantitative differences in drug affinity due to their different molecular basis.1,18 Another factor that complicates the use of zebrafish model in selection of candidate molecules for medicinal drug development or in toxicological testing is the total composition of inward and outward currents underlying the cardiac AP, which differs qualitatively (e.g, absence of ITo and IKs) and quantitatively (INa, ICaL) from that of the human heart. In risk/benefit assessment of new drug molecules, more emphasis is currently placed on the potential proarrhythmic effects of pharmaceuticals. According to the current research paradigm of safety pharmacology, proarrhythmic potential of the drugs should be look at the level of multiple ion channels (IKr, ICaL, INa, IKs and ITo) instead of one particular ion channel, since drug molecules may bind to several targets.45,48 In this experimental approach the total repolarizing reserve of outward currents and the total depolarizing reserves of inward currents is considered decisive in estimating the proarrhythmic liability of pharmaceuticals.27 Because of the qualitative and quantitative differences in inward and outward currents between zebrafish and human hearts, zebrafish heart and cardiac myocytes are likely to pose problems in nonclinical safety pharmacology and toxicology, especially in regard to arrhythmia liability of drugs.
Clearly, more research on molecular basis of cardiac ion channels, and ion channel regulation and drug sensitivity of cardiac ionic currents are needed to enable rational use of the zebrafish heart as an electrophysiological model for the human heart.
Methods
Animals
Zebrafish were reared at +28°C. All experiments were authorized by the National Animal Experimental Board in Finland (permission ESAVI/2832/04.10.07/2015).
Action potentials
Ventricular APs of spontaneously beating hearts were recorded with microelectrodes filled with 3M KCl (resistance about 20 mega ohms) in oxygenated external saline solution containing (in mM) NaCl 150, KCl 3.0, MgSO4 1.2, NaH2PO4 1.2, CaCl2 2.0, glucose 10.0 and HEPES 10.0 (at pH 7.7). Temperature of the tissue bath was changed at the rate of 3 degrees 10 min−1.
KCNH transcript expression
Atrium and ventricle of the heart were separately snap frozen in liquid nitrogen. Tissues from 5 fishes were pooled for each atrial and ventricular sample (n = 6) and stored at −80°C. RNA was extracted by TriReagent (Thermo Scientific) and treated with RNase free DNase to avoid DNA-contamination. First-strand cDNA and -RT-control (reaction containing all other components except the RT-enzyme) were synthesized from each sample using Maxima H Minus First Strand cDNA Synthesis Kit (Thermo Scientific). Each sample was amplified in triplicates using Maxima SYBR Green qPCR Master Mix (Thermo Scientific) (Table 3) and AriaMx Real Time PCR System (Agilent Technologies) under the cycling parameters: 95°C for 10 min followed by 40 cycles at 94°C for 10 s, 60°C for 20 s and 72°C for 30 s, then 72°C for 5 min. Specificity of amplification was monitored by melting curve analysis from 65°C to 95°C. Transcript levels of KCNH genes were normalized to the expression of DnaJA2 reference gene.
Table 3.
Primers used in qPCR.
| Gene | Primers (5′-3′) | Amplicon length (bp) |
|---|---|---|
| KCNH2A (ENSDARG00000029881) | F: TCTGTGATGGTGGACTGCTC R: CTGAGAGTGCGTTGAACGAG | 100 |
| KCNH2B (ENSDARG00000060053) | F: GGATCCGAAGACAGGAAACA R: TGATACTCCCTGCCTCGACT | 102 |
| KCNH6 (ENSDARG00000001803) | F: ATTCTCCCCTTCTGCAGTCA R: AGGGGCTGCTGTGTACTGAT | 100 |
| KCNH7 (ENSDARG00000062687) | F: CTTCACACACTGCCAGGAAA R: TGGTGTTCAGGTTGACAGGA | 100 |
Abbreviations
- AP
action potential
- hpf
hours post fertilization
- TTX
tetrodotoxin
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by a grant from Jane and Aatos Erkko Foundation (Finland) to MV. Dr. Dr. Vesa Paajanen is acknowledged for recording the human ECG.
References
- [1].Abi-Gerges N, Holkham H, Jones EM, Pollard CE, Valentin JP, Robertson GA. hERG subunit composition determines differential drug sensitivity. Br J Pharmacol 2011; 164:419–32; PMID:21449979; http://dx.doi.org/ 10.1111/j.1476-5381.2011.01378.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alday A, Alonso H, Gallego M, Urrutia J, Letamendia A, Callol C, Casis O. Ionic channels underlying the ventricular action potential in zebrafish embryo. Pharmacol Res 2014; 84:26–31; PMID:24747832; http://dx.doi.org/ 10.1016/j.phrs.2014.03.011 [DOI] [PubMed] [Google Scholar]
- [3].Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouzi M, Chi NC. Zebrafish model for human long QT syndrome. Proc Natl Acad Sci U S A 2007; 104:11316–21; PMID:17592134; http://dx.doi.org/ 10.1073/pnas.0702724104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arrenberg AB, Stainier DY, Baier H, Huisken J. Optogenetic control of cardiac function. Science 2010; 330:971–4; PMID:21071670; http://dx.doi.org/ 10.1126/science.1195929 [DOI] [PubMed] [Google Scholar]
- [5].Baker K, Warren KS, Yellen G, Fishman MC. Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc Natl Acad Sci U S A 1997; 94:4554–9; http://dx.doi.org/ 10.1073/pnas.94.9.4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bakkers J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc Res 2011; 91:279–88; PMID:21602174; http://dx.doi.org/ 10.1093/cvr/cvr098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Braasch I, Guiguen Y, Loker R, Letaw JH, Ferrara A, Bobe J, Postlethwait JH. Connectivity of vertebrate genomes: Paired-related homeobox (Prrx) genes in spotted gar, basal teleosts, and tetrapods. Comp Biochem Physiol C 2014; 163:24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brette F, Luxan G, Cros C, Dixey H, Wilson C, Shiels HA. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem Biophys Res Commun 2008; 374:143–6; PMID:18602892; http://dx.doi.org/ 10.1016/j.bbrc.2008.06.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chopra SS, Stroud DM, Watanabe H, Bennett JS, Burns CG, Wells KS, Yang T, Zhong TP, Roden DM. Voltage-gated sodium channels are required for heart development in zebrafish. Circ Res 2010; 106:1342–50; PMID:20339120; http://dx.doi.org/ 10.1161/CIRCRESAHA.109.213132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chopra SS, Watanabe H, Zhong TP, Roden DM. Molecular cloning and analysis of zebrafish voltage-gated sodium channel beta subunit genes: implications for the evolution of electrical signaling in vertebrates. BMC Evol Biol 2007; 7:113; PMID:17623065; http://dx.doi.org/ 10.1186/1471-2148-7-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cribbs LL, Lee J, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, et al.. Cloning and Characterization of a1H From Human Heart, a Member of the T-Type Ca2+ Channel Gene Family. Circ Res 1998; 83:103–9; PMID:9670923; http://dx.doi.org/ 10.1161/01.RES.83.1.103 [DOI] [PubMed] [Google Scholar]
- [12].Dahme T, Katus HA, Rottbauer W. Fishing for the genetic basis of cardiovascular disease. Dis Model Mech 2009; 2:18–22; PMID:19132116; http://dx.doi.org/ 10.1242/dmm.000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Eleawa SM, Sakr HF, Hussein AM, Assiri AS, Bayoumy NMK, Alkhateeb M. Effect of testosterone replacement therapy on cardiac performance and oxidative stress in orchidectomized rats. Acta Physiol 2013; 209:136–47; http://dx.doi.org/ 10.1111/apha.12158 [DOI] [PubMed] [Google Scholar]
- [14].Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J.Physiol. 2007; 582:675–93; http://dx.doi.org/ 10.1113/jphysiol.2006.126714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gosselin-Badaroudine P, Moreau A, Chahine M. Nav1.5 mutations linked to dilated cardiomyopathy phenotypes. Channels 2014; 8:90-4; http://dx.doi.org/ 10.4161/chan.27179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hassel D, Scholz EP, Trano N, Friedrich O, Just S, Meder B, Weiss DL, Zitron E, Marquart S, Vogel B, et al.. Deficient zebrafish ether-a-go-go-related gene channel gating causes short-QT syndrome in zebrafish reggae mutants. Circulation 2008; 117:866–75; PMID:18250272; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.107.752220 [DOI] [PubMed] [Google Scholar]
- [17].Hassinen M, Haverinen J, Hardy ME, Shiels HA, Vornanen M. Inward rectifier potassium current (IK1) and Kir2 composition of the zebrafish (Danio rerio) heart. Pflugers Arch 2015; 467:2437-46; http://dx.doi.org/ 10.1007/s00424-015-1710-8 [DOI] [PubMed] [Google Scholar]
- [18].Hassinen M, Haverinen J, Vornanen M. Molecular basis and drug sensitivity of the delayed rectifier (IKr) in the fish heart. Comp Biochem Physiol C Toxicol Pharmacol 2015; 176–177:44–51; PMID:26215639 [DOI] [PubMed] [Google Scholar]
- [19].Hassinen M, Haverinen J, Vornanen M. Electrophysiological properties and expression of the delayed rectifier potassium (ERG) channels in the heart of thermally acclimated rainbow trout. Am J Physiol 2008; 295:R297–308 [DOI] [PubMed] [Google Scholar]
- [20].Hassinen M, Laulaja S, Paajanen V, Haverinen J, Vornanen M. Thermal adaptation of the crucian carp (Carassius carassius) cardiac delayed rectifier current, IKs, by homomeric assembly of Kv7.1 subunits without MinK. Am J Physiol 2011; 301:R255–65; http://dx.doi.org/ 10.1152/ajpcell.00047.2011 [DOI] [PubMed] [Google Scholar]
- [21].Haverinen J, Hassinen M, Vornanen M. Fish cardiac sodium channels are tetrodotoxin sensitive. Acta Physiol 2007; 191:197–204; http://dx.doi.org/ 10.1111/j.1748-1716.2007.01734.x [DOI] [PubMed] [Google Scholar]
- [22].Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function and physiological role. Physiol Rev 2010; 90:291–366 [DOI] [PubMed] [Google Scholar]
- [23].Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, et al.. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013; 496:498–503; PMID:23594743; http://dx.doi.org/ 10.1038/nature12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hughes BA, Kumar G, Yuan Y, Swaminathan A, Yan D, Sharma A, Plumley L, Yang-Feng TL, Swaroop A. Cloning and functional expression of human retinal Kir2.4, a pH-sensitive inwardly rectifying K+ channel. Am J Physiol 2000; 279:C771–84 [DOI] [PubMed] [Google Scholar]
- [25].Huo R, Sheng Y, Guo W, Dong D. The potential role of Kv4.3 K+ channel in heart hypertrophy. 2014; 8:203–9; PMID:2476239725453103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jones DK, Liu F, Vaidyanathan R, Eckhardt LL, Trudeau MC, Robertson GA. hERG 1b is critical for human cardiac repolarization. Proc Natl Acad Sci U S A 2014; 111:18073–7; PMID:25453103; http://dx.doi.org/ 10.1073/pnas.1414945111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jost N, Virág L, Comtois P, Ördög B, Szuts V, Seprényi G, Bitay M, Kohajda Z, Koncz I, Nagy N, et al.. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J Physiol 2013; 591:4189–206; PMID:23878377; http://dx.doi.org/ 10.1113/jphysiol.2013.261198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Langheinrich U, Vacun G, Wagner T. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol 2003; 193:370–82; PMID:14678746; http://dx.doi.org/ 10.1016/j.taap.2003.07.012 [DOI] [PubMed] [Google Scholar]
- [29].Leong IU, Skinner JR, Shelling AN, Love DR. Zebrafish as a model for long QT syndrome: the evidence and the means of manipulating zebrafish gene expression. Acta Physiol (Oxf) 2010; 199:257–76; PMID:20331541 [DOI] [PubMed] [Google Scholar]
- [30].Leong IUS, Skinner JR, Shelling AN, Love DR. Expression of a mutant kcnj2 gene transcript in zebrafish. ISRN Mol Biol 2014; 324839:1–14; http://dx.doi.org/ 10.1155/2014/324839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Letamendia A, Quevedo C, Ibarbia I, Virto JM, Holgado O, Diez M, Belmonte JCI, Callol-Massot C. Development and validation of an automated high-throughput system for zebrafish in vivo screenings. 2012; 7:e36690; PMID:22615792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet 2007; 8:353–67; http://dx.doi.org/ 10.1038/nrg2091 [DOI] [PubMed] [Google Scholar]
- [33].Lin E, Ribeiro A, Ding W, Hove-Madsen L, Sarunic MV, Beg MF, Tibbits GF. Optical mapping of the electrical activity of isolated adult zebrafish hearts: acute effects of temperature. Am J Physiol 2014; 306:R823–36; http://dx.doi.org/ 10.1152/ajpcell.00423.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].López-Olmeda JF, Sánchez-Vázquez FJ. Thermal biology of zebrafish (Danio rerio). J Therm Biol 2011; 36:91–104; PMID:Can't; http://dx.doi.org/ 10.1016/j.jtherbio.2010.12.005 [DOI] [Google Scholar]
- [35].Lundquist AL, Manderfield LJ, Vanoye CG, Rogers CS, Donahue BS, Chang PA, Drinkwater DC, Murray KT Jr.. G. Expression of multiple KCNE genes in human heart may enable variable modulation of IKs. J Mol Cell Cardiol 2005; 38:277–87 [DOI] [PubMed] [Google Scholar]
- [36].Martinson AS, van Rossum DB, Diatta FH, Layden MJ, Rhodes SA, Martindale MQ, Jegla T. Functional evolution of Erg potassium channel gating reveals an ancient origin for IKr. Proc Natl Acad Sci U S A 2014; 111:5712–7; PMID:24706772; http://dx.doi.org/ 10.1073/pnas.1321716111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev 1994; 74:365–507; PMID:8171118 [DOI] [PubMed] [Google Scholar]
- [38].Melgari D, Brack KE, Zhang C, Zhang Y, El Harchi A, Mitcheson JS, Dempsey CE, Ng GA, Hancox JC. hERG potassium channel blockade by the HCN channel inhibitor bradycardic agent ivabradine. J Am Heart Assoc 2015; 4: 10.1161/JAHA.115.001813; PMID:25911606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs That Induce Repolarization Abnormalities Cause Bradycardia in Zebrafish. Circulation 2003; 107:1355–8 [DOI] [PubMed] [Google Scholar]
- [40].Nemtsas P, Wettwer E, Christ T, Weidinger G, Ravens U. Adult zebrafish heart as a model for human heart? An electrophysiological study. J Mol Cell Cardiol 2010; 48:161–71; PMID:19747484; http://dx.doi.org/ 10.1016/j.yjmcc.2009.08.034 [DOI] [PubMed] [Google Scholar]
- [41].Nerbonne JM. Studying Cardiac Arrhythmias in the Mouse - A Reasonable Model for Probing Mechanisms? Trends Cardiovasc Med 2004; 14:83–93; PMID:15121155; http://dx.doi.org/ 10.1016/j.tcm.2003.12.006 [DOI] [PubMed] [Google Scholar]
- [42].Novak AE, Taylor AD, Pineda RH, Lasda EL, Wright MA, Ribera AB. Embryonic and larval expression of zebrafish voltage-gated sodium channel alpha-subunit genes. Dev Dyn 2006; 235:1962–73; PMID:16615064; http://dx.doi.org/ 10.1002/dvdy.20811 [DOI] [PubMed] [Google Scholar]
- [43].Ono K, Iijima T. Cardiac T-type Ca2+ channels in the heart. J Mol Cell Cardiol 2010; 48:65–70; PMID:19729018; http://dx.doi.org/ 10.1016/j.yjmcc.2009.08.021 [DOI] [PubMed] [Google Scholar]
- [44].Priest BT, McDermott JS. Cardiac Ion. Channels 2015; 9:352-9; http://dx.doi.org/ 10.1080/19336950.2015.1076597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pugsley MK, Curtis MJ, Hayes ES. Biophysics and Molecular Biology of Cardiac Ion Channels for the Safety Pharmacologist. Handb. Exp Pharmacol 2015; 229:149–203; http://dx.doi.org/ 10.1007/978-3-662-46943-9_7 [DOI] [PubMed] [Google Scholar]
- [46].Rook MB, Evers MM, Vos MA, Bierhuizen MFA. Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc Res 2012; 93:12–23; PMID:21937582; http://dx.doi.org/ 10.1093/cvr/cvr252 [DOI] [PubMed] [Google Scholar]
- [47].Rottbauer W, Baker K, Wo ZG, Mohideen MPK, Cantiello HF, Fishman MC. Growth and function of the embryonic heart depend upon the cardiac-specific L-Type calcium channel alpha1 Subunit. Dev Cell 2001; 1:265–75; PMID:11702785; http://dx.doi.org/ 10.1016/S1534-5807(01)00023-5 [DOI] [PubMed] [Google Scholar]
- [48].Sager PT, Gintant G, Turner JR, Pettit S, Stockbridge N. Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the Cardiac Safety Research Consortium. Am Heart J 2014; 167:292–300; PMID:24576511; http://dx.doi.org/ 10.1016/j.ahj.2013.11.004 [DOI] [PubMed] [Google Scholar]
- [49].Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquird cardiac arrthytmia: HERG encodes the IKr potassium channel. Cell 1995; 81:299–307 [DOI] [PubMed] [Google Scholar]
- [50].Schmitt N, Grunnet M, Olesen SP. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev 2014; 94:609–53; PMID:24692356; http://dx.doi.org/ 10.1152/physrev.00022.2013 [DOI] [PubMed] [Google Scholar]
- [51].Scholz EP, Niemer N, Hassel D, Zitron E, Burgers HF, Bloehs R, Seyler C, Scherer D, Thomas D, Kathofer S, et al.. Biophysical properties of zebrafish ether-a-go-go related gene potassium channels. Biochem Biophys Res Commun 2009; 381:159–64; PMID:19232322; http://dx.doi.org/ 10.1016/j.bbrc.2009.02.042 [DOI] [PubMed] [Google Scholar]
- [52].Sidi S, Busch-Nentwich E, Friedrich R, Schoenberger U, Nicolson T. gemini encodes a zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. J Neurosci 2004; 24:4213–23; PMID:15115817; http://dx.doi.org/ 10.1523/JNEUROSCI.0223-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, Mohideen MA, Neuhauss SC, Solnica-Krezel L, Schier AF, et al.. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 1996; 123:285–92; PMID:9007248 [DOI] [PubMed] [Google Scholar]
- [54].Szuts V, Menesi D, Varga-Orvos Z, Zvara A, Houshmand N, Bitay M, Bogats G, Virag L, Baczko I, Szalontai B, et al.. Altered expression of genes for Kir ion channels in dilated cardiomyopathy. Can J Physiol Pharmacol 2013; 91:648–56; PMID:23889090; http://dx.doi.org/ 10.1139/cjpp-2012-0413 [DOI] [PubMed] [Google Scholar]
- [55].Tessadori F, van Weerd JH, Burkhard SB, Verkerk AO, de Pater E, Boukens BJ, Vink A, Christoffels VM, Bakkers J. Identification and functional characterization of cardiac pacemaker cells in zebrafish. PLoS One 2012; 7:e47644; PMID:23077655; http://dx.doi.org/ 10.1371/journal.pone.0047644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Verkerk AO, Remme CA. Zebrafish: a novel research tool for cardiac (patho)electrophysiology and ion channel disorders. Front Physiol 2012; 3:255; PMID:22934012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Volff JN. Genome evolution and biodiversity in teleost fish. Heredity 2005; 94:280–94 [DOI] [PubMed] [Google Scholar]
- [58].Vornanen M, Hassinen M, Haverinen J. Tetrodotoxin sensitivity of the vertebrate cardiac Na+ current. Mar Drugs 2011; 9:2409–22; PMID:22163193; http://dx.doi.org/ 10.3390/md9112409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA 1994; 91:3438–42; http://dx.doi.org/ 10.1073/pnas.91.8.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Warren KS, Baker K, Fishman MC. The slow mo mutation reduces pacemaker current and heart rate in adult zebrafish. Am J Physiol 2001; 281:H1711–9 [DOI] [PubMed] [Google Scholar]
- [61].Wong E, Yu WP, Yap WH, Venkatesh B, Soong TW. Comparative genomics of the human and Fugu voltage-gated calcium channel [alpha]1-subunit gene family reveals greater diversity in Fugu. Gene 2006; 366:117–27; PMID:16337095; http://dx.doi.org/ 10.1016/j.gene.2005.08.022 [DOI] [PubMed] [Google Scholar]
- [62].Wu C, Sharma K, Laster K, Hersi M, Torres C, Lukas TJ, Moore EJ. Kcnq1-5 (Kv7.1-5) potassium channel expression in the adult zebrafish. BMC Physiol 2014; 14:1; PMID:24555524; http://dx.doi.org/ 10.1186/1472-6793-14-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yu F, Zhao Y, Gu J, Quigley KL, Chi NC, Tai YC, Hsiai TK. Flexible microelectrode arrays to interface epicardial electrical signals with intracardial calcium transients in zebrafish hearts. Biomed Microdevices 2012; 14:357–66; PMID:22124886; http://dx.doi.org/ 10.1007/s10544-011-9612-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang C, Miki T, Shibasaki T, Yokokura M, Saraya A, Seino S. Identification and characterization of a novel member of the ATP-sensitive K+ channel subunit family, Kir6.3, in zebrafish. Physiol Genomics 2006; 24:290–7; PMID:16317080; http://dx.doi.org/ 10.1152/physiolgenomics.00228.2005 [DOI] [PubMed] [Google Scholar]
- [65].Zhang PC, Llach A, Sheng XY, Hove-Madsen L, Tibbits GF. Calcium handling in zebrafish ventricular myocytes. Am J Physiol 2011; 300:R56–66 [DOI] [PubMed] [Google Scholar]


