Phosphoinositides are minor amphipathic constituents of biological membranes. The most common phosphoinositide in the inner leaflet of the plasma membrane is phosphoinositol 4,5-bisphosphate (PI(4,5)P2), which forms ca. 1% of the total phospholipid pool of the membrane. PtdIns(4,5)P2 is not only a precursor of well-known second messengers such as IP3 and DAG, but also directly regulates several ion channels, including TRP channels.1 In recent years, many TRP channels were found to be positively regulated by PI(4,5)P2, although in some cases inhibitory effects were reported. In our recent work2 and in an independent study by the group of prof. Tibor Rohács in the Journal of General Physiology,3 we reported that TRPM3 is also tightly regulated by phosphoinositides. However, its specificity is toward PtdIns(4,5)P2 is much lower than what was found for other TRP channels, including other PI(4,5)P2-sensitive members of the TRPM subfamily.
Until a few years ago, TRPM3 was one of the less characterized members of the TRP superfamily, considered as a quite enigmatic channel having several transcript variants with unclear activation mechanism and functions. More recently, the channel entered the spotlight when it was identified as a steroid activated, heat sensitive ion channel that plays important roles in thermosensation and nociception in primary sensory neurons as well as in regulating insulin secretion in β cells.4,5 However, little is known about the cellular regulation of the channel.
In the highlighted papers, we provide the first information on how TRPM3 may be regulated at the cellular level by various signaling pathways, which may shape its function in sensory neurons or β cells. We demonstrate that enzymatic removal of PtdIns(4,5)P2 decreased the activity of TRPM3 in whole-cell patch-clamp measurements and in intact cells, whereas exogenous PI(4,5)P2 applied to the intracellular surface of the plasma membrane restored TRPM3 currents in inside-out patches. This finding links TRPM3 activity to metabotropic receptors that signal via PLC, such as histamine or bradykinin receptors, which are implicated in nociception and inflammation. However, the only partial effectiveness, even at high concentrations, of exogenous short chain PtdIns(4,5)P2 isoforms (compared with e.g. TRPM41) and the moderate effect of enzymatic PI(4,5)P2 degradation (compared with e.g., TRPM81) suggested that other factors beyond PtdIns(4,5)P2 influence channel activity. For instance, we found that the length of the acyl chain in PIPs has a significant effect on the PI(4,5)P2 mediated current recovery, probably influencing the membrane integration of the exogenous PtdIns(4,5)P2. More interestingly, we observed that PI(3,4,5)P3 is the most effective PIP in activating TRPM3, with a potency that far exceeds that of PtdIns(4,5)P2 (Fig. 1). This low PIP selectivity, and in particular the high PI(3,4,5)P3 preference of TRPM3 is unique among TRP channels. Whether PtdIns(3,4,5)P3 acts as a positive cofactor under physiological circumstances is unclear, since PI(3,4,5)P3 levels in the membrane of resting cells are believed to be too low to have a meaningful effect on channel activity. It will be of great interest to investigate whether signaling events that lead to increased membrane PtdIns(3,4,5)P3 levels, in particular receptors that cause activation of phosphoinositol 3-kinase (PI3K), regulate the activity of TRPM3 in vitro and in vivo.
Figure 1.
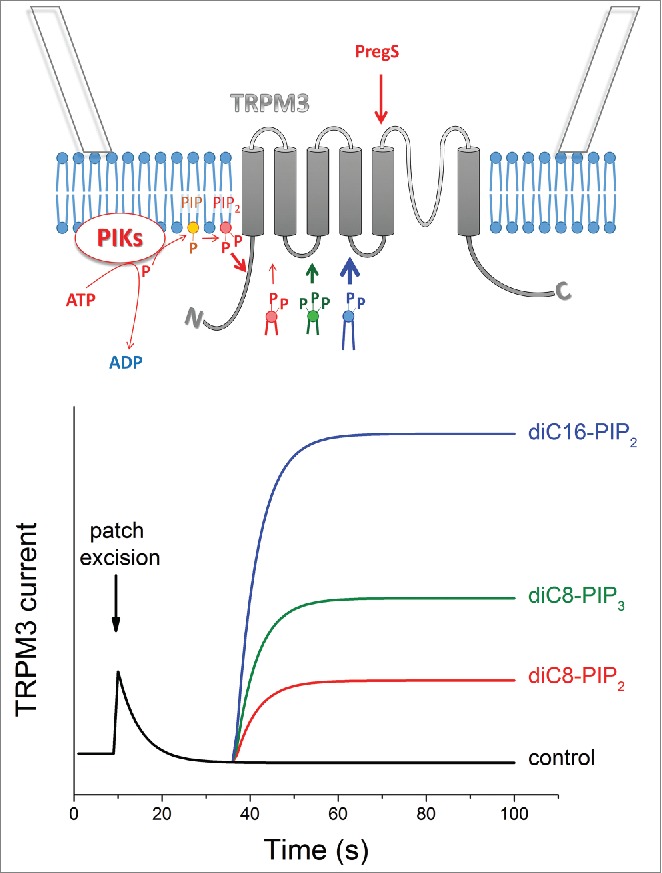
Phosphoinositols regulate TRPM3. ATP and exogenous PIPs applied to the cytoplasmic surface restore the TRPM3 current after desensitization in inside-out membrane patches. ATP was found to restore the PIP2 level in the plasma membrane. The efficacy of exogenous PIPs depends on the negatively charged polar head group and the length of the acyl chains, as well. For more details see the text. Abbreviations: PIKs – phosphatidylinositol kinases, PIP – phosphatidylinositol (mono)phosphate, PIP2 – phosphatidylinositol bisphosphate, diC8 PIP2 – dioctanoyl phosphatidylinositol bisphosphate, diC8 PIP3 – dioctanoyl phosphatidylinositol trisphosphate, diC16 PIP2 – dipalmitoyl phosphatidylinositol bisphosphate, PregS – pregnenlone sulfate.
Another unresolved question is the location of the binding site(s) for the PIPs on TRPM3. In the TRPM subfamily we can find examples where the binding sites are located in the C-terminal TRP box, such as TRPM81 or TRPM6.6 In contrast, putative C-terminal PH-like domains were identified as PI(4,5)P2 binding sites in TRPM4.1 Regarding TRPM3, the only data were presented in the pages of “Channel” in the form of an Addendum paper, in which Holendova and colleagues showed that protein constructs containing N-terminal fragments of the human TRPM3, which bind Ca2+-calmodulin and S100A1, can bind to PtdIns(4,5)P2 as well.7 This result argues for the existence of a PI(4,5)P2 binding site outside of the C-terminal TRP box, but its nature and exact location remains unknown.
In our recent work, wild type TRPM3 was found to exhibit an alternative pore analogous to the gating pore/omega pore in mutant voltage-gated channels, with potential importance for its sensory functions.8 To our knowledge, there are no data concerning PIP regulation of such gating pores in any ion channels. An interesting outstanding question is whether the physically separate alternative pore in TRPM3 is regulated in a similar manner to the main central pore.
Overall, these studies allow us to include TRPM3 in the list of channels and transporters regulated by PIPs. Our findings raise several exciting questions, both on the molecular (“What determines PIP selectivity in TRP channels?”) and (patho)physiological level(“Do PIPs regulate TRPM3 to tune sensory sensitivity or insulin secretion?”).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. EMBO J 2008; 27:2809-16; PMID:18923420; http://dx.doi.org/ 10.1038/emboj.2008.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Toth BI, Konrad M, Ghosh D, Mohr F, Halaszovich CR, Leitner MG, Vriens J, Oberwinkler J, Voets T. Regulation of the transient receptor potential channel TRPM3 by phosphoinositides. J Gen Physiol 2015; 146:51-63; PMID:26123194; http://dx.doi.org/ 10.1085/jgp.201411339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Badheka D, Borbiro I, Rohacs T. Transient receptor potential melastatin 3 is a phosphoinositide-dependent ion channel. J Gen Physiol 2015; 146:65-77; PMID:26123195; http://dx.doi.org/ 10.1085/jgp.201411336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011; 70:482-94; PMID:21555074; http://dx.doi.org/ 10.1016/j.neuron.2011.02.051 [DOI] [PubMed] [Google Scholar]
- [5].Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Dufer M, Lis A, Flockerzi V, Philipp SE, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol 2008; 10:1421-30; PMID:18978782; http://dx.doi.org/ 10.1038/ncb1801 [DOI] [PubMed] [Google Scholar]
- [6].Xie J, Sun B, Du J, Yang W, Chen HC, Overton JD, Runnels LW, Yue L. Phosphatidylinositol 4,5-bisphosphate (PIP(2)) controls magnesium gatekeeper TRPM6 activity. Sci Rep 2011; 1:146; PMID:22180838; http://dx.doi.org/ 10.1038/srep00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Holendova B, Grycova L, Jirku M, Teisinger J. PtdIns(4,5)P2 interacts with CaM binding domains on TRPM3 N-terminus. Channels (Austin) 2012; 6:479-82; PMID:22989896; http://dx.doi.org/ 10.4161/chan.22177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vriens J, Held K, Janssens A, Toth BI, Kerselaers S, Nilius B, Vennekens R, Voets T. Opening of an alternative ion permeation pathway in a nociceptor TRP channel. Nat Chem Biol 2014; 10:188-95; PMID:24390427; http://dx.doi.org/ 10.1038/nchembio.1428 [DOI] [PubMed] [Google Scholar]


