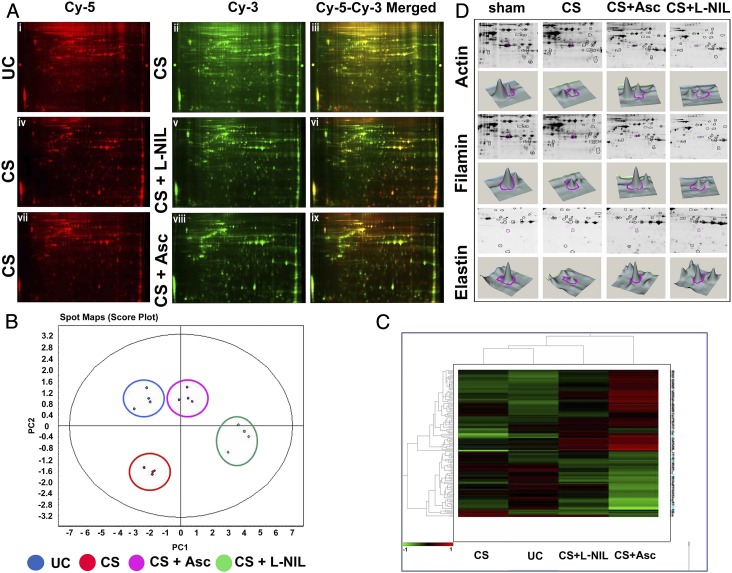
Fig. 5.
Ascorbate prevents proteolysis of human lung proteins in normal human lung epithelial cells treated with CSE. (A) DIGE analysis shows lung protein degradation on CS exposure and comparative prevention achieved through ascorbate and L-NIL pretreatment. The first row depicts the comparative DIGE profiles of the CS-untreated (Cy-5 red, i) lung epithelial proteins from the NL-20 cells against their CS-treated counterparts (Cy-3, green, ii) along with their corresponding overlay (Cy-5–Cy-3 yellow, iii). The second and third rows similarly depict the comparative DIGE profiles of the respective CS-treated (Cy-5 red, iv and vii) against their CS + L-NIL (Cy-3 green, v) and CS + Asc (Cy-3 green, viii) treated counterparts along with their corresponding overlays (Cy-3–Cy-5 yellow, vi and ix). Replicate spot maps of the above four experimental groups were filtered with ANOVA < 0.001 and subjected to DeCyder EDA with PCA (B) and hierarchical clustering (C). (D) CS-induced degradation of lung proteins identified by DeCyder followed by MALDI-TOF analysis. Among many proteins, three important lung structural proteins, namely actin (2.14-fold decrease; P < 0.01), filamin (1.77-fold decrease; P = 0.0053) and elastin (2.4-fold decrease; P < 0.01), were identified to undergo extensive degradation and were protected by ascorbate but not L-NIL.