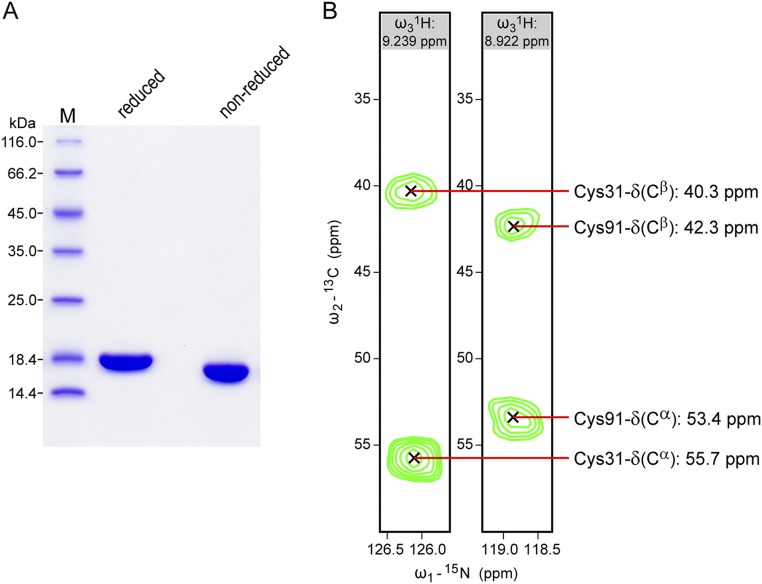
Fig. S7.
Purity of recombinant Siglec-8 lectin domain and presence of the intradomain disulfide bond (between Cys31 and Cys91) assessed by SDS/PAGE and NMR spectroscopy. (A) Coomassie blue-stained 15%-SDS/PAGE of purified Siglec-8 (natural isotopic composition) under reduced and nonreduced conditions. The slightly faster migration of nonreduced versus reduced protein, and the appearance of the nonreduced protein as a single band clearly indicate the uniform presence of the intradomain disulfide-bond. M, molecular weight marker. (B) Strips of a 3D CBCA(CO)NH spectrum of 13C/15N-labeled Siglec-8 at the 1H-15N resonances of Ser32 and Ser92 reveal the 13C chemical shifts of the Cα and Cβ atoms of their preceding residues Cys31 and Cys91, respectively. The observed 13C chemical shift frequencies are within the characteristic ranges for oxidized cysteines (62), confirming their involvement in a disulfide bond.