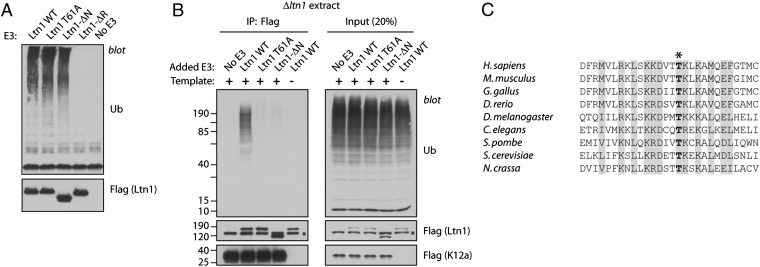
Fig. 3.
Ltn1 requires an intact N terminus for NSP ubiquitylation. (A) The conserved Ltn1 N-terminal domain (NTD) is not required for autoubiquitylation activity. In vitro ubiquitylation assay containing E. coli-expressed E1, E2 (Ubc4), GST-Ub, and C-terminal flag-tagged yeast Ltn1. The Ltn1 proteins used were WT (amino acids 1–1562), NTD-deleted mutant (Ltn1-ΔN; amino acids 477–1562), T61A mutant, and RING domain-deleted mutant (Ltn1-ΔR; amino acids 1–1502). (B) An intact NTD is required for Ltn1 function in NSP ubiquitylation. fGFP-K12–encoding mRNA (500 ng; full-length GFP used) was translated in Δltn1 extract [50 μL supplemented with 40 nM recombinant Ltn1 (WT or mutants, as in “A”) for 30 min] and analyzed for ubiquitylation by denaturing flag IP as in Fig. 1B. The control reaction in lanes 5 lacked fGFP-K12 template mRNA. (C) Alignment showing evolutionary conservation of Thr61 (bold, asterisk) and neighboring residues (46–73 in yeast) in selected Ltn1 orthologs.