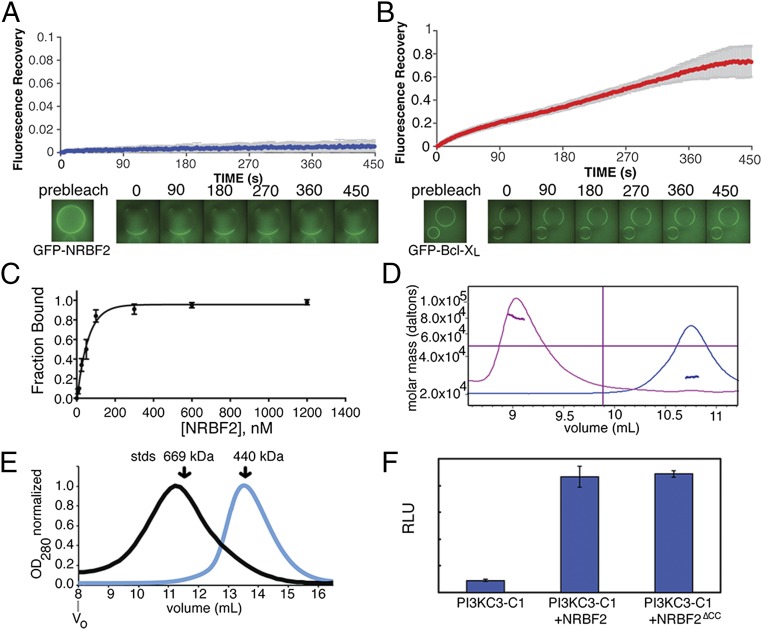
Fig. 1.
NRBF2 is a tightly bound subunit that activates and dimerizes PI3KC3-C1. (A) Quantitation of FRAP of GFP-NRBF2 bound to PI3KC3-C1. Recovery was monitored at 2-s intervals for 450 s. PI3KC3-C1 is bound to StrepTactin resin through streptavidin protein binding tags on PI3KC3-C1. Averages and SD of three replicates are shown. (B) Quantitation of FRAP of GFP- Bcl-XL∆TM bound to SBP-PI3KC3-C1; recovery was monitored for 450 s. PI3KC3-C1 is bound to Strep-Tactin resin through streptavidin protein binding tags on PI3KC3-C1. Averages and SD of three replicates are shown. (C) Binding curve of the average of three independent binding assays, displayed with the SEM, shows the binding affinity to be 40 nM. (D) MALS of full-length NRBF2 (pink trace) and NRBF2∆CC (blue trace). Full-length NRBF2 is 78 kDa and NRBF2∆CC is 27 kDa. (E) SEC with PI3KC3-C1+NRBF2 (black trace) and PI3KC3-C1 (blue trace). The peak shifts from 13.6 mL to 11.2 mL on a Superose 6 10/30 column. Molecular weight standards are indicated. (F) In vitro activity assay using sonicated liposomes containing phosphatidylinositol with PI3KC3-C1 and, where indicated, NRBF2 or NRBF2∆CC. RLU, relative light units.