Figure 2.
Design of a Stable hAChE variant and Its Functional Expression in Bacteria
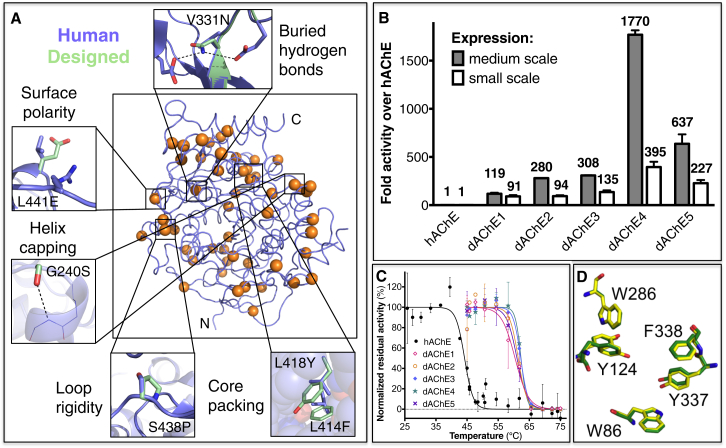
(A) The structural underpinnings of stabilization in the designed variant dAChE4. Wild-type hAChE is shown in blue and 51 mutated positions, which are distributed throughout dAChE4, are indicated by orange spheres. Thumbnails highlight stabilizing effects of selected mutations.
(B) Bacterial lysate activity levels of designed AChEs normalized to hAChE activity. Crude lysates were derived from 250 ml flasks (medium scale) or 0.5 ml E. coli cultures grown in a 96-well plate (small scale). The higher activity levels in the designed variants reflect higher levels of soluble, functional enzyme.
(C) Designed AChE variants (colored lines) show higher resistance to heat inactivation compared to hAChE (black). Residual activities following incubation at different temperatures were measured in bacterial lysates and normalized to the activity in nontreated lysates.
(D) Sub-Ångstrom accuracy in alignment of key residues in the vicinity of the catalytic triad in the crystallographic structure of dAChE4 (PDB: 5HQ3, yellow) compared to hAChE (PDB: 4EY4, green).
See also Figure S1 and Tables S2–S5.