Figure 3.
Higher Expression, Stability, and Activity of Designed Variants of PTE, SIRT6, Dnmt3a, and Myoc-OLF
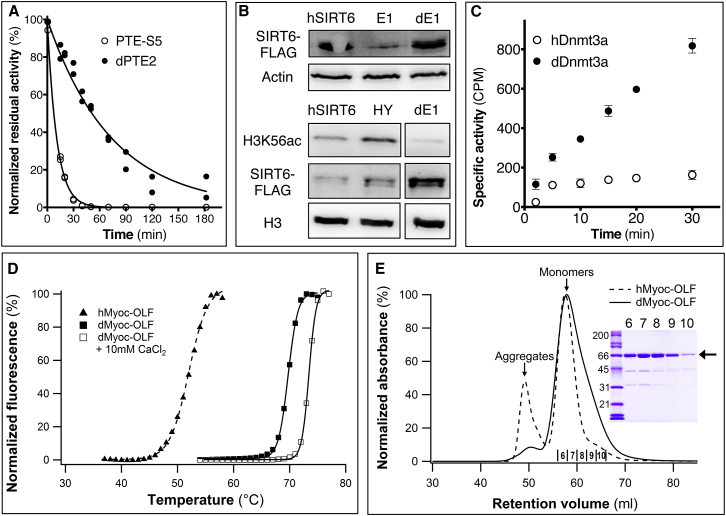
(A) Compared to the previously engineered variant PTE-S5 (Roodveldt and Tawfik, 2005), the design dPTE2 shows higher resistance to inactivation by the metal chelator 1,10-phenanthroline (50 μM), indicating higher metal affinity and stability.
(B) (Upper panel) The previously engineered E1 variant of hSIRT6 shows a 3-fold decline in in vivo expression levels, whereas following computational design, dSIRT6-E1 (denoted as dE1) recapitulates hSIRT6’s expression levels (western blot quantified using ImageJ). Actin expression levels are provided as control. (Lower panel) dE1 exhibits higher in vivo histone H3 Lys56-deacylation activity compared to hSIRT6. HY denotes a loss-of-function mutant of hSIRT6; H3 expression levels are provided as control.
(C) The designed variant dDnmt3A shows 10-fold higher DNA-methylation compared to hDnmt3a as determined by the levels of incorporation of H3-methyl groups in the presence of equal enzyme concentrations.
(D) dMyoc-OLF is more thermostable than hMyoc-OLF, and addition of Ca+2 further stabilizes it.
(E) Size-exclusion chromatography of MBP-fused hMyoc-OLF and dMyoc-OLF indicated a significant aggregated fraction in hMyoc-OLF, as previously described (Burns et al., 2010), and a minor aggregated fraction in dMyoc-OLF. (Inset) SDS-PAGE analysis of dMyoc-OLF. Lane 1, molecular weight standards (kDa); lanes 2–6, size exclusion fractions as labeled in chromatogram.
See also Figures S2 and S3 and Tables S2, S3, and S6.