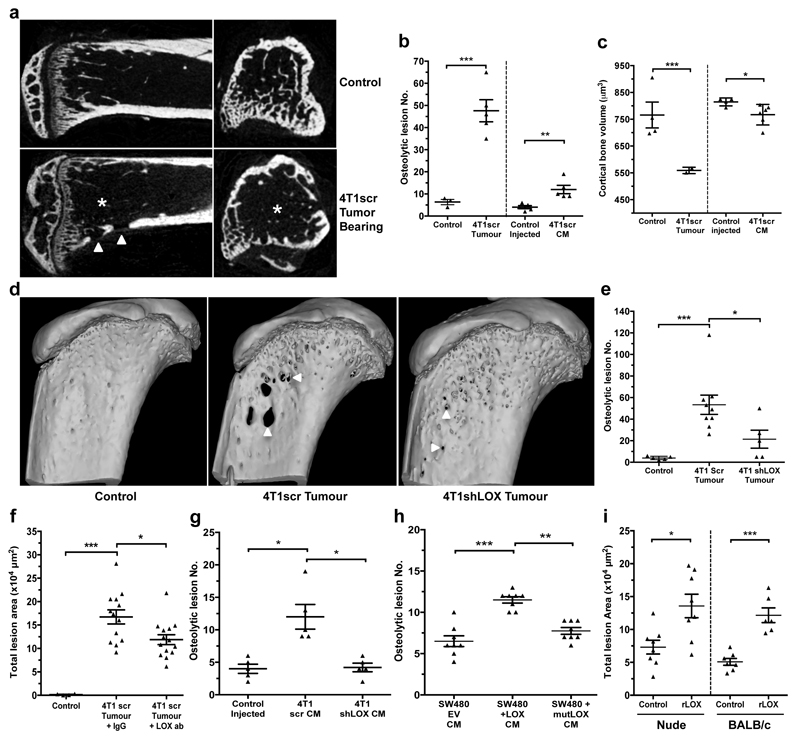
Figure 2. Osteolytic lesion formation in ER– mammary carcinoma models is LOX dependent.
a, Representative 2D cross-sections of tibia from control (top) and tumour-bearing (bottom) mice 3-weeks post orthotopic implantation showing lesions (arrowheads) and loss of trabecular structure (asterisks). b, Micro-CT analysis of osteolytic lesions in tumour-bearing and tumour-free, CM conditioned mice at 3 weeks (n: mice; control 3; 4T1scr Tumour 5; Control Injected 5; 4T1scr CM 5) c, Loss of cortical bone volume in 4T1scr tumour-bearing and tumour-free CM injected models at 3 weeks (n: mice; control 4; 4T1scr Tumour 3; Control Injected 4; 4T1scr CM 6). d, Representative 3D reconstructions of tibiae showing tumour driven osteolytic lesions (arrowheads). e, LOX silencing decreases focal osteolytic lesion formation (n: mice; control 5; 4T1scr Tumour 8; 4T1 shLOX Tumour 5). f, LOX inhibition decreases osteolytic lesion formation in tumour-bearing models (n: mice; control 5; 4T1scr Tumour + IgG 13; 4T1scr Tumour + LOX Ab 14) and g, in tumour-free CM injection models (n: mice; control 5; 4T1scr CM 5; 4T1shLOX CM 5) h, SW480 human CRC lines with stably manipulated LOX expression (EV, +LOX or +mutLOX) confirms LOX-dependency (n=8 mice per condition). i, Exogenous recombinant LOX (rLOX) drives osteolytic lesion formation in Nude and BALB/c models (n: mice; Nude control 8; Nude rLOX 8; BALB/c control 7; BALB/c rLOX 6). (b,c,e,f,g,h,i) Data shown are mean ± SEM. P-values derived from unpaired parametric one-tailed t-tests. (*P<0.05, **P<0.01, ***P<0.001).