Abstract
Objectives
The usual practice in breast cancer screening programmes for mammogram interpretation is to perform double reading. However, little is known about its cost-effectiveness in the context of digital mammography. Our purpose was to evaluate the cost-effectiveness of double reading versus single reading of digital mammograms in a population-based breast cancer screening programme.
Methods
Data from 28,636 screened women was used to establish a decision-tree model and to compare three strategies: 1) double reading; 2) double reading for women in their first participation and single reading for women in their subsequent participations; and 3) single reading. We calculated the incremental cost-effectiveness ratio (ICER), which was defined as the expected cost per one additionally detected cancer. We performed a deterministic sensitivity analysis to test the robustness of the ICER.
Results
The detection rate of double reading (5.17‰) was similar to that of single reading (4.78‰; P = .768). The mean cost of each detected cancer was €8,912 for double reading and €8,287 for single reading. The ICER of double reading versus single reading was €16,684. The sensitivity analysis showed variations in the ICER according to the sensitivity of reading strategies. The strategy that combines double reading in first participation with single reading in subsequent participations was ruled out due to extended dominance.
Conclusions
From our results, double reading appears not to be a cost-effective strategy in the context of digital mammography. Double reading would eventually be challenged in screening programmes, as single reading might entail important net savings without significantly changing the cancer detection rate. These results are not conclusive and should be confirmed in prospective studies that investigate long-term outcomes like quality adjusted life years (QALYs).
Introduction
Mammogram is the test of choice in European breast cancer screening programmes since it can detect breast cancer at an early stage [1–3]. Whereas digital mammography is a technology that can reduce false-positive results, no significant differences in the cancer detection rate were stated when it was compared to screen-film mammography [4]. In addition, an evaluation of its costs showed that screening with digital mammography can save long-term budget expense in breast cancer screening programmes [5]. Screening with digital mammography, therefore, has been widely implemented.
As two readers are unaware of each other’s interpretation, double reading can increase sensitivity reducing the chance of missed lesions [6–12]. Thus, double reading of digital mammograms became the usual practice in European programmes [1]. However, the following reasons might bring into question its cost-effectiveness.
The effectiveness of double reading may be less important in situations where a high level of agreement between radiologists exists [13]. The benefit of double reading may be restricted to particular settings in which cancer detection is difficult, i.e. mammograms of women in their first participation (prevalent screening) when no previous images are available, women with small lesions that are not easy to find, or when the readers are less experienced [6,14–17]. In addition, having two readers may significantly increase the time, staff costs and resources used in the reading process [18].
Information obtained from cost-effectiveness analyses is useful to decision makers when deciding to implement breast cancer screening programmes and evaluate its benefits and potential harms. One previous cost-effectiveness analysis based on European data, reported that risk-based strategies could reduce harms and costs [19]. Shifting from double reading to single reading was not analysed in this study. However, it is reasonable to hypothesize that in some contexts more benefits can be obtained from single reading as it may reduce costs and false-positives without significantly reducing the cancer detection rate [20]. Conversely, other cost-effectiveness analyses performed in European countries reported double reading as a cost-effective strategy in programmes that used screen-film mammography [1,6,21,22],
Recently, economic evaluations have focused on the cost-effectiveness of double reading versus the combination of single reading and CAD (computer-aid detection) [23–26], whilst studies of single reading without CAD have not yet been published in the context of digital mammography [23,27]. In fact, as further as we know, little is known about whether double reading is a cost-effective strategy in digital screening. Therefore, the main purpose of this study was to assess the cost-effectiveness of double reading versus single reading of digital mammograms in a breast cancer screening programme.
Materials and Methods
Study population
This study was performed in women participating in a population-based breast cancer screening programme of the Hospital Sant Pau in an area of 390,000 inhabitants in Barcelona, Spain. The programme was funded by the Public Health Insurance to invite women of 50–69 years of age for biennial screening. In this study, we included all digital mammograms performed from June 2009 until May 2011 and followed up until May 2013. We excluded two mammograms because the data of the reader’s interpretation was incomplete. A total of 28,636 mammograms were analysed, 5,978 (20.9%) corresponding to women participating for the first time in the breast cancer screening programme (prevalent screening), and 22,658 (79.1%) corresponding to subsequent participations (subsequent screening) (Table 1).
Table 1. Characteristics of the women included in the analysis.
| Participants in one screening round (2009–2011) | ||
|---|---|---|
| No. | % | |
| Study population¶ | 28,636 | 100.0 |
| Prevalent screening | 5,978 | 20.9 |
| Incident screening | 22,658 | 79.1 |
| Age at screening | ||
| 50–54 | 8,181 | 28.6 |
| 55–59 | 6,947 | 24.3 |
| 60–64 | 7,047 | 24.6 |
| 65–69 | 6,461 | 22.6 |
¶The information of these women was included in the decision-tree model as common parameters for all reading strategies.
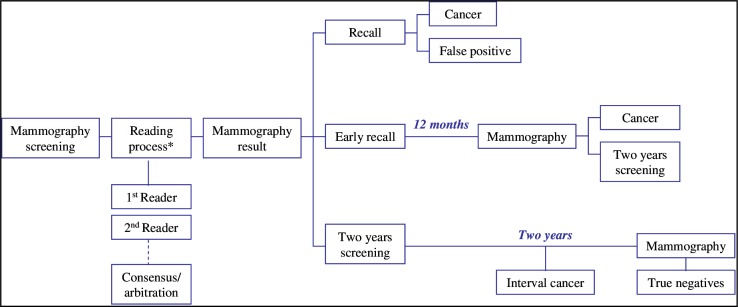
Two projections (mediolateral-oblique and craniocaudal) were taken per breast in all screening examinations. Four certified screening radiologists, who read at least 5,000 mammograms per year, read the mammograms in the breast cancer screening programme. Two radiologists separately read each mammogram (independent double reading). The radiologists classified the results of each mammogram as follows: (1) recall, in which case additional tests were requested to confirm or ruled out malignancy; (2) early recall, in which case the woman was programmed for a further screening mammography in 12 months; or (3) two-year screening, in which case the woman was programmed for a further screening mammography in two years. In case of disagreement between radiologists, the result of the mammogram was determined by consensus or by arbitration. Fig 1 shows the algorithm of the decisions made in a screening round.
Fig 1. Algorithm followed during a biennial screening round in the programme.
*The reading process included independent double reading followed by consensus and arbitration in case of disagreement.
Screen-detected cancers were pathologically confirmed breast cancers, both ductal carcinoma in situ (DCIS) and invasive carcinomas. We did not include breast malignancies other than primary breast cancers. The cancer detection rate was calculated as the number of screen-detected cancers divided by the number of participants. The Ethics Committee of the Hospital Sant Pau approved the study. Informed consent was not required, since the data were retrospectively collected, and records were anonymized before receipt and analysis.
Model design
A decision-tree model was used because of its appropriateness to reflect the immediate effects of decisions taken during a screening round. In this model, the main outcome of effectiveness was the screen-detected cancers; other performance and diagnostic accuracy outcomes were also analysed (S1 Table). The time horizon was four years–from June 2009 to May 2013–, which included a biennial screening round (2009–2011) plus two-year follow-up to confirm negative results. This model assumes that participants do not suffer other conditions that prevent them from successfully completing the entire time horizon.
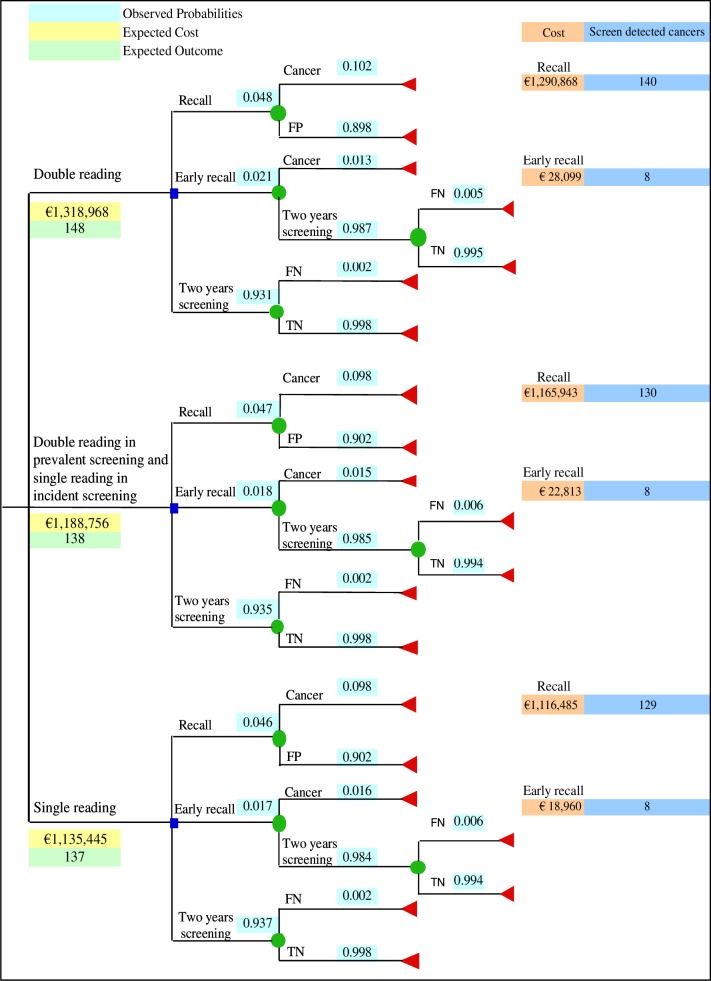
The cost-effectiveness of double versus single reading was evaluated comparing the following strategies (Fig 2):
Fig 2. Decision-tree model used to evaluate the cost-effectiveness of the three reading strategies.
FP = False Positive. FN = False Negative. TN = True Negative.
Double reading
Double reading with consensus and arbitration was the procedure performed in the breast cancer screening programme. For this strategy, we included the results of mammograms (recall, early recall or two-year screening) in the model, as well as the number of cancers in the way they were observed in a real setting.
Double reading in prevalent screening and single reading in incident screening
This strategy was based on the assumption that double reading was performed only for mammograms of women in their first participation, and single reading was performed for mammograms of women in their subsequent participations.
Single reading
This strategy was based on the assumption that the first radiologist alone determined the result of mammograms. In order to evaluate the effect of switching from the first to the second reader, we included the results of the second radiologist in the sensitivity analysis.
Costs data
As this study was conducted from the perspective of the public health system, we included direct healthcare costs of practitioner and diagnostic tests. Total costs were calculated using a bottom-up costing method in two stages: mammography screening and additional tests. Unitary costs for the first stage were obtained from the programme database. The financial department at the hospital provided costs for the second stage. All costs were calculated in Euro (€) (2010 value) (S2 Table). Since long-term health benefits are expected in a preventive programme, NICE’s recommendation carries a lower discount rate that reflects society’s preferences for benefits in the future [28–30]. In this study, we used a short-term horizon that prevents important variations associated with discounting. For these two reasons, no discount rate has been applied.
Determining cost-effectiveness
We compared reading strategies using incremental cost-effectiveness ratio (ICER) that indicates the additional cost of obtaining one additionally screen detected cancer. To construct the hierarchy of cost-effectiveness of the reading strategies we did the following: firstly, we calculated the average cost-effectiveness ratio (ACER) by dividing the total costs of each strategy by its corresponding number of screen detected cancers. This allowed us to ruled out, when necessary, a dominated strategy (more costly and less effective than alternatives). Secondly, we calculated ICERs and reported them starting with the lowest additional cost per additionally detected cancer. This allowed us to identify a strategy with lower effectiveness and higher ICER and to rule it out for extended dominance. Finally, ICERs for the non-ruled out strategies were compared. The most cost-effective strategy showed the lowest ICER.
Sensitivity analysis
A deterministic sensitivity analysis was carried out to assess the robustness of ICERs according to the variation in the following variables: participation rate, breast cancer prevalence, sensitivity of the reading strategy, positive-predictive value (PPV) of recall, early recall rate and staff costs (S3 Table).
Statistical tests were two-sided and all P values of less than 0.05 were considered statistically significant. We used Microsoft Excel, Redmond, Washington (2011) in all analyses.
Results
Effectiveness of reading strategies
The characteristics of the 28,636 women included in the study are presented in Table 1. The most relevant data for comparing the three reading strategies are the following. The number of screen detected cancers of the three strategies was: 148 (5.17‰) for double reading, 138 (4.82‰) for double reading in prevalent screening and single reading in incident screening, and 137 (4.78‰) for single reading (P = 0.768). Sixteen interval cancers (0.56‰) occurred after double reading (Table 2).
Table 2. Effectiveness-outcomes of reading strategies included in the model.
| Double reading | Double reading in prevalent screening and single reading in incident screening | Single reading | P value | ||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Mammogram results | |||||||
| Recall | 1,366 | 4.8 | 1,333 | 4.7 | 1,322 | 4.6 | .004 |
| Early recall† | 609 | 2.1 | 523 | 1.8 | 490 | 1.7 | |
| Two-year screening | 26,661 | 93.1 | 26,780 | 93.5 | 26,824 | 93.7 | |
| Performance measure | |||||||
| Readers’ agreement | 27,022 | 94.4 | 27,982 | 97.7 | NA | NA | < .001 |
| Consensus or arbitration | 1,614 | 5.6 | 654 | 2.3 | NA | NA | |
| PPV of recall | 140 | 10.2 | 130 | 9.8 | 129 | 9.8 | .884 |
| Diagnostic accuracy | |||||||
| Sensitivity | 148 | 94.8% | 138 | 88.5% | 137 | 87.8% | .027∑ |
| Specificity | 27,214 | 95.5% | 27,242 | 95.6% | 27,256 | 95.7% | .389∑ |
| Cancers | |||||||
| Cancer detection rate¶ | 148 | 5.17‰ | 138 | 4.82‰ | 137 | 4.78‰ | .768 |
| Interval cancers§ | 16 | 0.56‰ | 26 | 0.91‰ | 27 | 0.94‰ | .200 .093∑ |
| Histologic type | |||||||
| Invasive | 116 | 79.5 | 111 | 81.6 | 111 | 82.2 | .822 |
| In situ | 30 | 20.5 | 25 | 18.4 | 24 | 17.8 | |
| Unknown | 2 | - | 2 | - | 2 | - | |
NA = not applicable. PPV = positive predictive value.
†Women referred to mammography-control in 12 months.
∑Chi square test of double versus single reading.
¶Detection rate per 1000 screened women.
§Interval cancers detected in the subsequent two years after a negative screening episode.
Characteristics of screen detected cancers
The number of screen detected cancers at double reading was higher in incident screening (n = 104) than in prevalent screening (n = 44). However, compared to incident screening (4.59‰), the cancer detection rate was higher in prevalent screening (7.36‰; P = 0.008). Of the 148 screen detected cancers, 117 (79.1%) were detected in concordance between both radiologists, while 31 (20.9%) were detected after consensus or arbitration. Thirty (20.5%) were carcinomas in situ (CIS) and 116 (79.5%) were invasive carcinomas. Compared to single reading, double reading increased by 25% (n = 6) and 4.5% (n = 5) the number of CIS and invasive carcinomas, respectively. No other relevant differences were observed in the characteristics of screen detected cancers according to reading strategies (S3 Table).
Cost of reading strategies and additional diagnostic tests
The cost of the three strategies was the following: €1,318,968 for double reading; €1,188,756 for double reading in prevalent screening and single reading in incident screening; and €1,135,445 for single reading. The final amount of common costs was €855,298, which was 64.9% and 75.3% of the total costs at double and single reading, respectively. Differences in non-common costs were mostly attributed to the number of readings and additional diagnostic tests (Table 3).
Table 3. Costs estimation for a biennial period (2009–2011) according to reading strategy.
| Common parameters for all three reading strategies | |||||||
| No. | Cost € | ||||||
| Mammogram | 28,636 | 164,787 | |||||
| Staff | NA | 690,510 | |||||
| Non-common costs | Double reading | Double reading in prevalent screening and single reading in incident screening | Single reading | ||||
| No. | Cost € | No. | Cost € | No. | Cost € | ||
| ßStaff | NA | 255,363 | NA | 156,874 | NA | 123,052 | |
| ¶Additional tests | 1,414 | 205,170 | 1376 | 173,890 | 1361 | 154,570 | |
| Supplies in early recall | 609 | 2,331 | 523 | 2,002 | 490 | 1,876 | |
| *Maintenance in early recall | NA | 807 | NA | 693 | NA | 649 | |
| Total | 1,318,968 | 1,188,756 | 1,135,445 | ||||
NA = not apply.
ßRadiologist and administrative staff involved in the reading process, consensus or arbitration; also administrative and technical staff involved in the early recall process.
¶Ultrasound, additional mammography, fine-needle aspiration cytology, core biopsy, open surgical biopsy, other minimal procedures.
*Including depreciation of mammography machine.
Cost effectiveness analysis
Average cost-effectiveness ratio
The average cost of each screen detected cancer for the three strategies was €8,912 for double reading; €8,614 for double reading in prevalent screening and single reading in incident screening; and €8,287 for single reading. Compared to double reading, the other two strategies were less expensive and less effective; therefore, none of the strategies was ruled out because of dominance.
Incremental cost-effectiveness ratio (ICER)
Table 4 shows the process to calculate ICERs. The ICER of double reading in prevalent screening and single reading in incident screening versus single reading was €53,312. This strategy was ruled out of the analysis due to extended dominance. The ICER of double versus single reading was €16,684. This amount was more than twice the average cost per one cancer detected at single reading (€8,287).
Table 4. Process to calculate the Incremental Cost-Effectiveness Ratio (ICER) of the reading strategies.
| Reading strategy | Expected cost € | Expected outcome | Incremental cost € | Incremental effect | Average cost € | ICER € | |
|---|---|---|---|---|---|---|---|
| No. of cancers | Detection rate | ||||||
| Single reading | 1,135,445 | 137 | 4.78‰ | 8,287 | |||
| Double reading in prevalent screening and single reading in incident screening | 1,188,756 | 138 | 4.82‰ | 53,312 | 1 | 8,614 | 53,312 |
| Double reading | 1,318,968 | 148 | 5.17‰ | 183,523 | 11 | 8,912 | ¶16,684 |
¶The strategy that combines double reading in prevalent screening with single reading in incident screening was ruled out by extended dominance; therefore, the value of the ICER represents the comparison between double and single reading.
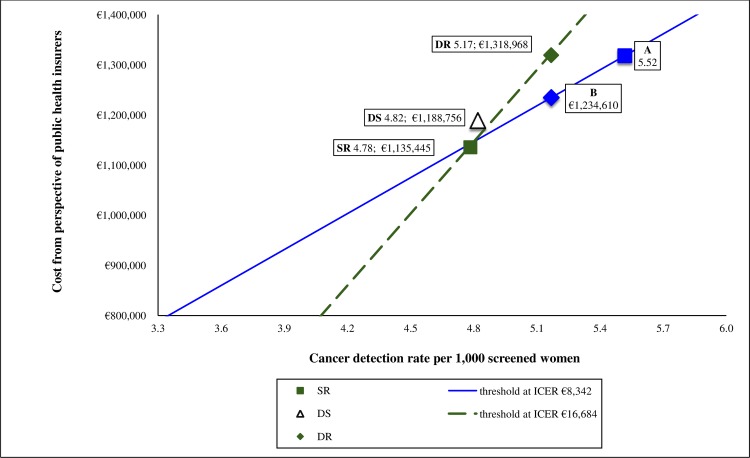
In Fig 3, the ICERs are plotted over the cost-effectiveness plane where the continuous line represents the expected performance at single reading. The discontinuous line shows that shifting from single to double reading resulted in an increment of €183,523 in cost, and an increase of 0.39‰ in the cancer detection rate. In order to be as cost-effective as single reading, the required cancer detection rate and cost of double reading should shift to points A and B, respectively.
Fig 3. Cost-effectiveness plane illustrating differences in costs and cancer detection rates between the reading strategies.
DR = Double reading. SR = Single reading. DS = Double reading in prevalent screening and single reading in incident screening. Continuous and dashed lines represent the thresholds if willingness to pay per one additionally detected cancer were €8,342 and €16,684, respectively. Point A represents the expected cancer detection rate at single reading if willingness to pay per single reading were equal to double reading. Point B represents the expected cost at single reading if the cancer detection rate at single reading were equal to that of double reading.
Sensitivity analysis
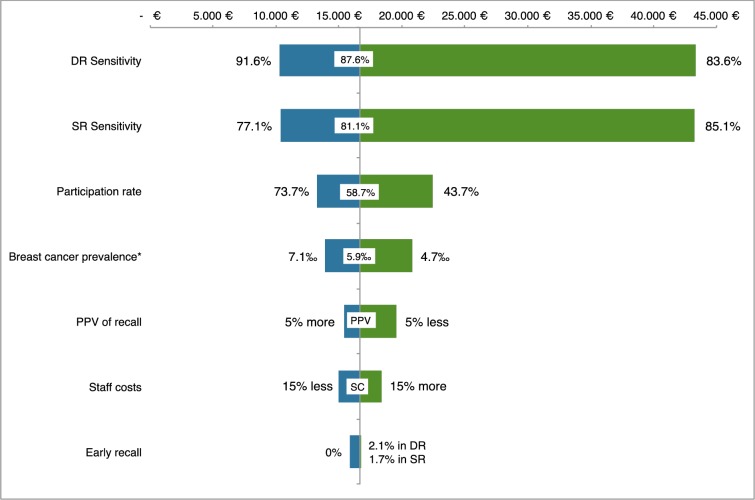
Our model was more sensitive to changes in terms of sensitivity of reading strategies for detecting cancers. Varying the current participation rate and observed breast cancer prevalence also affected our results, whereas changes in PPV of recall, staff costs or early recall proportion had less impact on the results (Fig 4 and S5 Table).
Fig 4. Sensitivity analysis of the incremental cost-effectiveness ratio (ICER) of double reading versus single reading.
DR = Double reading. SR = Single reading. PPV = positive predictive value. SC = Staff costs. ER = early recall was 2.1% in double reading and 1.7% in single reading. *The prevalence of breast cancer was estimated as the number of true positives plus the number of false negatives.
Discussion
We propose a decision-tree model to compare three reading strategies in a population-based breast cancer screening programme. The results of this cost-effectiveness analysis showed that the ICER per one additionally detected cancer was drastically higher (100% more expensive) for double reading than the average cost of each screen detected cancer at single reading. To our knowledge, this is one of the first economic evaluations of double reading versus single reading in the context of digital mammography [18], and the first one assessing single reading without CAD [27].
Our results might be useful for exploring those strategies that require less expensive ICER per one additionally screen detected cancer. Firstly, we found that the combination of double reading in prevalent screening and single reading in incident screening was not cost-effective due to extended dominance, which means that its ICER was higher than that of double reading. This result can be explained by the characteristics of cancers in incident screening that are frequently detected after discordance between readers [14]. Secondly, the effectiveness to detect cancers was similar between double reading and single reading. In fact, an agreement between readers’ interpretations was observed in 94.4% of mammograms and the cancer detection rate of double reading increased only by 8%, as reported in a systematic review [6]. Thirdly, the ICER (€16,684) of double reading was approximately 100% more expensive than the average cost of one screen detected cancer at single reading. Therefore, double reading might not be as cost-effective as single reading.
Aside from the ICER, characteristics of additionally detected cancers after double reading might inform about the potential benefits of this reading strategy in breast cancer screening programmes. European guidelines recommend to limit the proportion of detected ductal carcinomas in-situ (DCIS) [1], due to its potential association with overdiagnosis. On the other hand, there is evidence that DCIS detection reduces interval cancer rates [31]. Based on a Spanish cohort, Blanch et al. [15] reported that double reading had a greater effect on detection of CIS. Similarly, we found that detection of CIS increased by 25% at double reading. Although currently there is not evidence that screening programmes should take measures to reduce DCIS detection, further studies will focus on the association between reading strategies and overdiagnosis.
In our study, both lower recall and early recall rates at single reading show important cost savings due to a reduction in additional diagnostic tests. This net benefit was not at the expense of decreasing its positive predictive value (PPV), which remained stable when comparing it with double reading. Our results match with The US National Cancer Institute findings that showed a better interpretive accuracy and higher PPV at single reading [31].
The ICER at double reading remained rather stable in the sensitivity analysis. However, a small change (5%) in the sensitivity of double reading or single reading could carry important variations in the ICER. To be as cost-effective as single reading, detection rate at double reading should increase by 16%. On the contrary, a reduction in staff cost was not key determinant of ICER variations. Because the parameters we used to test uncertainties reflect the European guidelines’ recommendations [1], we believe that our results might provide reasonable estimates to be extrapolated to other screening programmes with similar characteristics.
In contrast with our results, previous studies comparing double versus single reading reported that double reading was a cost-effective strategy [6,22]. This discrepancy can be explained by the fact that those studies were performed in the context of screen-film mammography. Digital mammography can be more sensitive than screen-film mammography, reducing the possibility of missed lesions. Furthermore, previous studies were performed decades ago in the firsts rounds of screening programmes when costs were probably lower than nowadays. Our results came from the fifth round of a well-established programme when the high experience of one single reader can be as good as the combination of two readers.
Although the scientific evidence is insufficient to determine the benefits of CAD on the readers interpretation [23,32,33]. Sato et al. [24] found that single reading + CAD was a cost-effective strategy increasing life years gained at a ‘low’ price. Our results cannot be comparable with those published by Sato et al. [24] because we did not evaluate CAD and we did not have evidence of life-year gained with single reading. However, the results of both studies may stimulate rethinking of single reading alone or single reading + CAD as a feasible and efficient strategy in settings where shortage of radiologists exists.
The study has certain limitations. First, we did not assess the quality-adjusted life years (QALYs) for each strategy. Although there is no universal agreement, QALY is currently the most appropriate measure of health benefit, and interventions are usually considered cost-effective when the ICER placed on a QALY gained is up to €30,000 [28]. Nevertheless, our results might be useful for calculating expected costs per one additionally screen detected cancer and, therefore, can be taken into account when planning or evaluating breast cancer screening programmes. Second, it is difficult to compare our results with published cost-effectiveness evaluations [18] because of known differences in programmes between countries. However, our results may be similar to other European breast cancer screening programmes. Third, several economic evaluations have reported superiority of single reading with CAD over double reading [23,24]. We did not evaluate this new technology because it is not widely available in Spain. Fourth, although there is no statistical difference in detection rate between single and double reading, this study may not be adequately powered for the small expected difference. Finally, in this study we included information about five highly trained radiologists. The results can be different in other context with less trained radiologists or other professionals performing the reading of mammograms.
In conclusion, our results suggest that, in the context of digital mammography, double reading double reading can be as effective as single reading but more expensive. Whereas double reading did not dramatically increase the cancer detection rate, the current question is how much the decision makers are willing to pay for the extra cancers detected. Further economic evaluation of randomised controlled trials may be crucial in determining whether the QALYs gained at double reading are comparable to those at single reading in the context of digital mammography.
Supporting Information
(DOCX)
NA = not apply. ¶Staff = medical, technician, and administrative. *Maintenance and repairs of the machine including depreciation. ßAdministrative staff in early recall, as well as in consensus and arbitration. ∑In cancers cases suspected by one or both readers but with missing data, the average cost of additional tests was imputed.
(DOCX)
(DOCX)
¶Detection rate per 1000 screened women. ‡Linear test. §Interval cancers detected in the subsequent two years after a negative screening episode.
(DOCX)
FN = false negatives.
(DOCX)
Acknowledgments
The authors thank Ms. María Victoria Leo Rosas, for her contributions to the language editing of the present article. The authors also thank members of the Screening Mammography Program of Hospital Sant Pau, Barcelona, and participants in the programme. Margarita Posso is a Ph.D. candidate at the Methodology of Biomedical Research and Public Health program, Universitat Autònoma de Barcelona (UAB), Barcelona, Spain.
Data Availability
All relevant data are within the paper and its Supporting Information files. Additional data are available upon request from the authors.
Funding Statement
The authors have no support or funding to report.
References
- 1.Perry N, Puthaar E. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis. European Communities; 2006. [DOI] [PubMed] [Google Scholar]
- 2.Broeders M, Moss S, Nyström L, Njor S, Paap E, Massat N, et al. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. 2012;19 Suppl 1: 14–25. 10.1258/jms.2012.012078 [DOI] [PubMed] [Google Scholar]
- 3.The Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. 2012;380: 1778–1786. 10.1016/S0140-6736(12)61611-0 [DOI] [PubMed] [Google Scholar]
- 4.Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353: 1773–1783. 10.1056/NEJMoa052911 [DOI] [PubMed] [Google Scholar]
- 5.Comas M, Arrospide A, Mar J, Sala M, Vilaprinyo E, Hernández C, et al. Budget impact analysis of switching to digital mammography in a population-based breast cancer screening program: a discrete event simulation model. PLoS ONE. 2014;9: e97459 10.1371/journal.pone.0097459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinnes J, Moss S, Melia J, Blanks R, Song F, Kleijnen J. Effectiveness and cost-effectiveness of double reading of mammograms in breast cancer screening: findings of a systematic review. The Breast. 2001;10: 455–463. 10.1054/brst.2001.0350 [DOI] [PubMed] [Google Scholar]
- 7.Blanks RG, Wallis MG, Moss SM. A comparison of cancer detection rates achieved by breast cancer screening programmes by number of readers, for one and two view mammography: results from the UK National Health Service breast screening programme. J Med Screen. 1998;5: 195–201. 10.1136/jms.5.4.195 [DOI] [PubMed] [Google Scholar]
- 8.Harvey SC, Geller B, Oppenheimer RG, Pinet M, Riddell L, Garra B. Increase in cancer detection and recall rates with independent double interpretation of screening mammography. American Journal of Roentgenology. 2003;180: 1461–1467. [DOI] [PubMed] [Google Scholar]
- 9.Matson M, Hibbert J, Field S. Double reporting of screening mammograms. Clin Radiol. 1997;52: 567 [DOI] [PubMed] [Google Scholar]
- 10.Thurfjell EL, Lernevall KA, Taube AA. Benefit of independent double reading in a population-based mammography screening program. Radiology. 1994;191: 241–244. 10.1148/radiology.191.1.8134580 [DOI] [PubMed] [Google Scholar]
- 11.Thurfjell E. Mammography screening. One versus two views and independent double reading. Acta Radiol. 1994;35: 345–350. [PubMed] [Google Scholar]
- 12.Klompenhouwer EG, Voogd AC, Heeten den GJ, Strobbe LJA, de Haan AFJ, Wauters CA, et al. Blinded double reading yields a higher programme sensitivity than non-blinded double reading at digital screening mammography: a prospected population based study in the south of The Netherlands. Eur J Cancer. 2015;51: 391–399. 10.1016/j.ejca.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 13.Taplin SH, Rutter CM, Elmore JG, Seger D, White D, Brenner RJ. Accuracy of screening mammography using single versus independent double interpretation. American Journal of Roentgenology. 2000;174: 1257–1262. 10.2214/ajr.174.5.1741257 [DOI] [PubMed] [Google Scholar]
- 14.Hofvind S, Geller BM, Rosenberg RD, Skaane P. Screening-detected breast cancers: discordant independent double reading in a population-based screening program. Radiology. 2009;253: 652–660. 10.1148/radiol.2533090210 [DOI] [PubMed] [Google Scholar]
- 15.Blanch J, Sala M, Román M, Ederra M, Salas D, Zubizarreta R, et al. Cumulative risk of cancer detection in breast cancer screening by protocol strategy. Breast Cancer Res Treat. 2013;138: 869–877. 10.1007/s10549-013-2458-5 [DOI] [PubMed] [Google Scholar]
- 16.Roelofs AAJ, Karssemeijer N, Wedekind N, Beck C, van Woudenberg S, Snoeren PR, et al. Importance of comparison of current and prior mammograms in breast cancer screening. Radiology. 2007;242: 70–77. 10.1148/radiol.2421050684 [DOI] [PubMed] [Google Scholar]
- 17.Varela C, Karssemeijer N, Hendriks JHCL, Holland R. Use of prior mammograms in the classification of benign and malignant masses. Eur J Radiol. 2005;56: 248–255. 10.1016/j.ejrad.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 18.Leivo T, Salminen T, Sintonen H, Tuominen R, Auerma K, Partanen K, et al. Incremental cost-effectiveness of double-reading mammograms. Breast Cancer Res Treat. 1999;54: 261–267. [DOI] [PubMed] [Google Scholar]
- 19.Vilaprinyo E, Forné C, Carles M, Sala M, Pla R, Castells X, et al. Cost-effectiveness and harm-benefit analyses of risk-based screening strategies for breast cancer. PLoS ONE. 2014;9: e86858 10.1371/journal.pone.0086858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posso MC, Puig T, Quintana MJ, Solà-Roca J, Bonfill X. Double versus single reading of mammograms in a breast cancer screening programme: a cost-consequence analysis. Eur Radiol. 2016. 10.1007/s00330-015-4175-4 [DOI] [PubMed] [Google Scholar]
- 21.Ciatto S, Ambrogetti D, Bonardi R, Catarzi S, Risso G, Rosselli Del Turco M, et al. Second reading of screening mammograms increases cancer detection and recall rates. Results in the Florence screening programme. J Med Screen. 2005;12: 103–106. 10.1258/0969141053908285 [DOI] [PubMed] [Google Scholar]
- 22.Groenewoud JH, Otten JDM, Fracheboud J, Draisma G, van Ineveld BM, Holland R, et al. Cost-effectiveness of different reading and referral strategies in mammography screening in the Netherlands. Breast Cancer Res Treat. 2007;102: 211–218. 10.1007/s10549-006-9319-4 [DOI] [PubMed] [Google Scholar]
- 23.Azavedo E, Zackrisson S, Mejàre I, Heibert Arnlind M. Is single reading with computer-aided detection (CAD) as good as double reading in mammography screening? A systematic review. BMC Med Imaging. 2012;12: 22 10.1186/1471-2342-12-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato M, Kawai M, Nishino Y, Shibuya D, Ohuchi N, Ishibashi T. Cost-effectiveness analysis for breast cancer screening: double reading versus single + CAD reading. Breast Cancer. Springer Japan; 2014;21: 532–541. 10.1007/s12282-012-0423-5 [DOI] [PubMed] [Google Scholar]
- 25.Guerriero C, Gillan MGC, Cairns J, Wallis MG, Gilbert FJ . Is computer aided detection (CAD) cost effective in screening mammography? A model based on the CADET II study. BMC Health Serv Res. 2011;11: 11 10.1186/1472-6963-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert FJ, Astley SM, Gillan MGC, Agbaje OF, Wallis MG, James J, et al. Single reading with computer-aided detection for screening mammography. N Engl J Med. 2008;359: 1675–1684. 10.1056/NEJMoa0803545 [DOI] [PubMed] [Google Scholar]
- 27.Taylor P, Potts HWW. Computer aids and human second reading as interventions in screening mammography: two systematic reviews to compare effects on cancer detection and recall rate. Eur J Cancer. 2008;44: 798–807. 10.1016/j.ejca.2008.02.016 [DOI] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013 [Internet]. London: NICE; 2014. Available: http://publications.nice.org.uk/pmg9 [PubMed] [Google Scholar]
- 29.O’Mahony JF, Paulden M. NICE's selective application of differential discounting: ambiguous, inconsistent, and unjustified. Value Health. 2014;17: 493–496. 10.1016/j.jval.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 30.Paulden M, O’Mahony JF, Culyer AJ, McCabe C. Some Inconsistencies in NICE’s Consideration of Social Values. PharmacoEconomics. 2014;32: 1043–1053. 10.1007/s40273-014-0204-4 [DOI] [PubMed] [Google Scholar]
- 31.Taplin S, Abraham L, Barlow WE, Fenton JJ, Berns EA, Carney PA, et al. Mammography facility characteristics associated with interpretive accuracy of screening mammography. J Natl Cancer Inst. 2008;100: 876–887. 10.1093/jnci/djn172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eadie LH, Taylor P, Gibson AP. A systematic review of computer-assisted diagnosis in diagnostic cancer imaging. Eur J Radiol. 2012;81: e70–6. 10.1016/j.ejrad.2011.01.098 [DOI] [PubMed] [Google Scholar]
- 33.Bargalló X, Santamaría G, Del Amo M, Arguis P, Ríos J, Grau J, et al. Single reading with computer-aided detection performed by selected radiologists in a breast cancer screening program. Eur J Radiol. Elsevier; 2014;0. 10.1016/j.ejrad.2014.08.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
NA = not apply. ¶Staff = medical, technician, and administrative. *Maintenance and repairs of the machine including depreciation. ßAdministrative staff in early recall, as well as in consensus and arbitration. ∑In cancers cases suspected by one or both readers but with missing data, the average cost of additional tests was imputed.
(DOCX)
(DOCX)
¶Detection rate per 1000 screened women. ‡Linear test. §Interval cancers detected in the subsequent two years after a negative screening episode.
(DOCX)
FN = false negatives.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Additional data are available upon request from the authors.