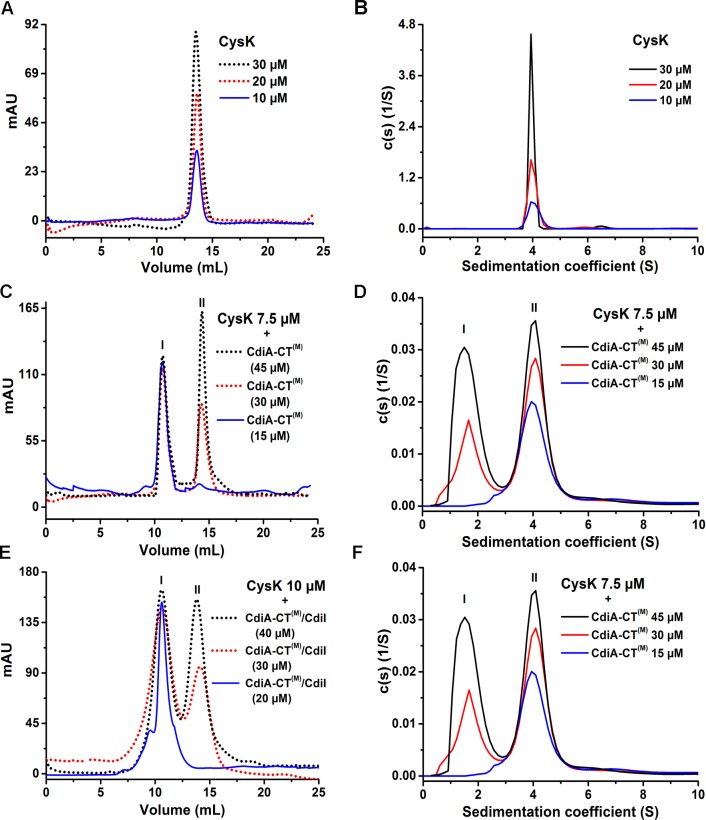
Fig 3. SEC and AUC analyses of the CysK, CdiA-CT(M)/CysK and CdiA-CT(M)/CdiI/CysK complexes.
(A) and (B) Both SEC and AUC data suggests dimeric state of CysK. (C) CdiA-CT(M)/CysK complex elutes as a single peak when mixed 0.5:1 molar of CysK dimer:CdiA-CT(M). Peak I, CdiA-CT(M)/CysK complex; peak II, excess CdiA-CT(M) (D) AUC analysis suggests 2:2 stoichiometric complex of CdiA-CT (M)/CysK. Peaks I and II corresponds to CdiA-CT(M) and CdiA-CT(M)/CysK complex, respectively. (E) The stoichiometric homogenous ternary complex is formed when preformed CdiA-CT(M)/CdiI complex is mixed with dimeric CysK in 1:0.5 molar ratio. Peak I, CdiA-CT(M)/CdiI/CysK complex; peak II, excess CdiA-CT(M)/CdiI complex. (F) CdiA-CT(M)/CdiI/CysK forms 2:2:2 stoichiometric complex as suggested by AUC. Peak I, CdiA-CT(M)/CdiI complex; peak II CdiA-CT(M)/CdiI/CysK ternary complex. In all the above experiments dimeric CysK concentration is used.