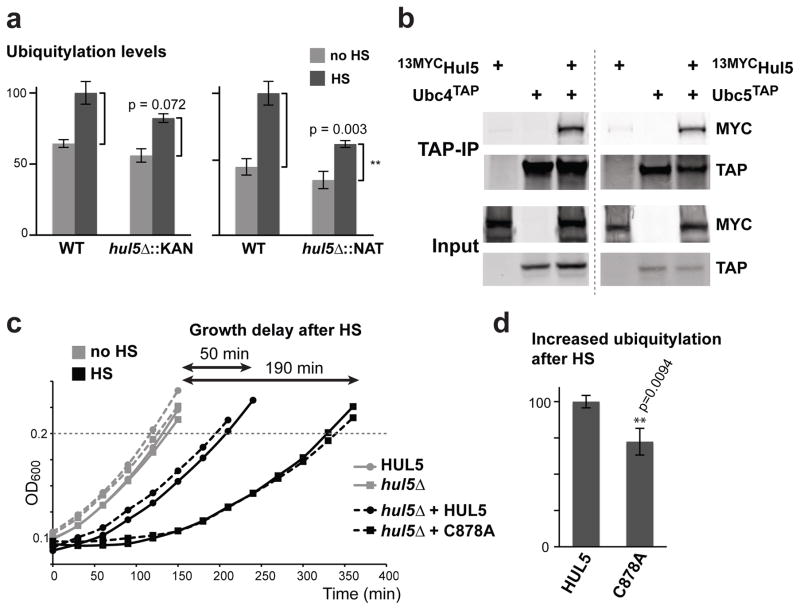
Figure 3.
HUL5 is required for the full ubiquitylation response and cell fitness after heat-shock. (a) Ubiquitylation levels in unstressed (no HS) and heat-shocked (HS) cells are compared between wild-type and hul5Δ strains using the dot blot assay. HUL5 was deleted by two different cassettes (KanMX6 and NatMX4). Standard deviation is shown for three replicates and p values were calculated using an unpaired Student’s t-test. (b) TAP pull-down experiments with cells expressing or not Ubc4TAP (left panel) or Ubc5TAP (right panel) at endogenous levels with or without a plasmid expressing 13MycHul5 were analyzed by Western blot using 9E10 or anti-TAP antibodies. Inputs (1%) are shown below. (c) The OD600 of the first cell doubling following a 20 min incubation at 45°C heat-shock (HS; black) or at 25°C (no HS; grey) of HUL5 (round) and hul5Δ::NAT (square) cells. hul5Δ::NAT cells carrying a centromeric plasmid (dashed lines) with HUL5 (round) or hul5-C878A (square) are also compared. Growth delay is defined by the difference of the first doubling time between the corresponding unstressed and stressed cells. Each data point is averaged from three replicates. (d) hul5Δ cells carrying either wild-type HUL5 or the catalytically inactive Hul5 (C878A) were subjected to heat-shock (15 min at 42°C). The increase in ubiquitylation levels (with standard deviations) was measured by dot blots with anti-ubiquitin and anti-Pgk1 antibodies in three replicates. An unpaired Student’s t-test was used to assess the significance of the difference between both cell populations.