Figure 2. Crizotinib-resistance in a ROS1 NSCLC patient.
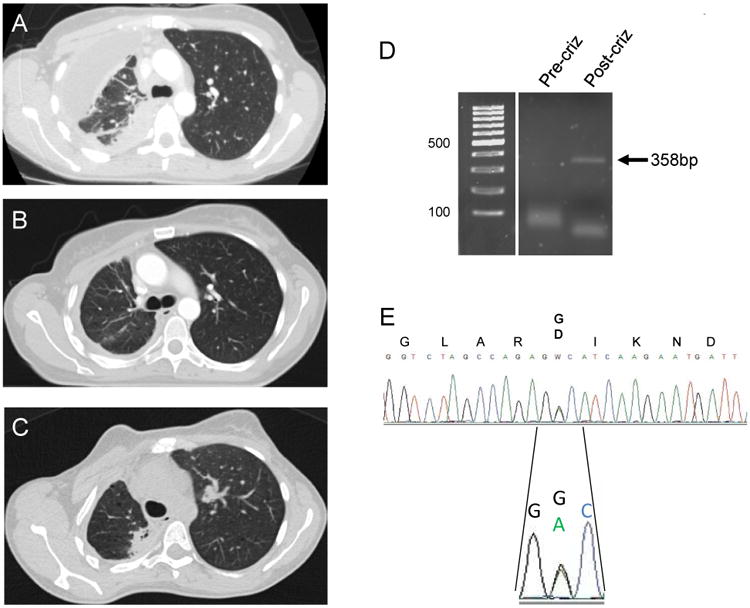
Computed tomography (CT) scans demonstrating pre-treatment disease (A), response to therapy on crizotinib (B) and disease-progression on crizotinib (C) in a ROS1+ NSCLC patient. (D) Reverse transcriptase polymerase-chain (RT-PCR) reaction of a region of the KIT kinase domain in pre- and post-crizotinib treatment tumor biopsies. The correct-sized PCR product is indicated by an arrow and is only observed in the post-crizotinib tumor sample. (E) Sanger DNA sequencing of the RT-PCR product observed in (D), demonstrating the presence of the mutation c.2447A>G encoding the KIT p.D816G amino acid substitution. Expression of both the wildtype and mutant KIT mRNA was observed.
