Figure 1.
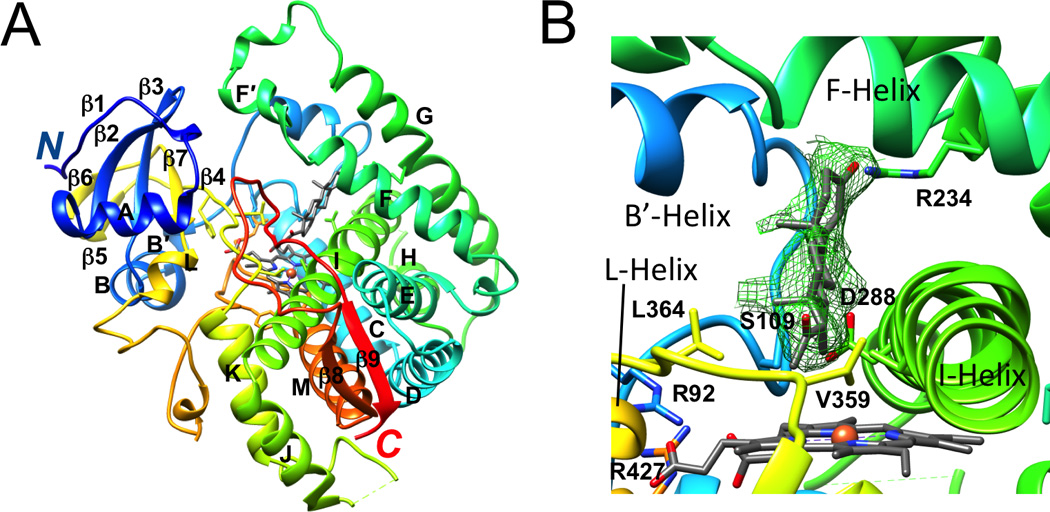
Tertiary structure of the P450 21A2 progesterone complex and active site configuration (PDB 4Y8W; www.rcsb.org) [28]. (A) Ribbon diagram of the overall fold of P450 21A2 with rainbow coloring from blue (N-terminus) to red (C-terminus). Individual secondary structural elements are labeled; carbon atoms of heme and progesterone are colored in gray; Fe3+ is shown as an orange sphere. Membrane P450s feature at least 12 α-helices that are designated with letters A to L and an N-terminal β-sheet domain with consecutive numbering of strands [27]. The protein core comprises helices C, D, I, K, and L and the β-sheets that make up part of the heme-binding site and the adjacent region where partner proteins dock. Substrates bind in a cleft above the heme, with several channels opening to the surface. The catalytic cysteine is located on the other side of heme, such that its thiolate moiety occupies the axial coordination site of iron opposite the bound oxygen. Helices B, C, F, and G that are situated more on the periphery of the protein and form the outer boundaries of the substrate cavity exhibit more structural variations and can be expected to display a more dynamic behavior as do the N-and C-terminal ends. (B) Close-up view of the active site with progesterone surrounded by Fourier 2Fo-Fc omit electron density drawn at the 1.2 σ threshold. Selected amino acids and α-helices wrapping around heme or forming the ceiling of the active site are labeled. All images were generated with the program UCSF Chimera [29].
