Figure 5.
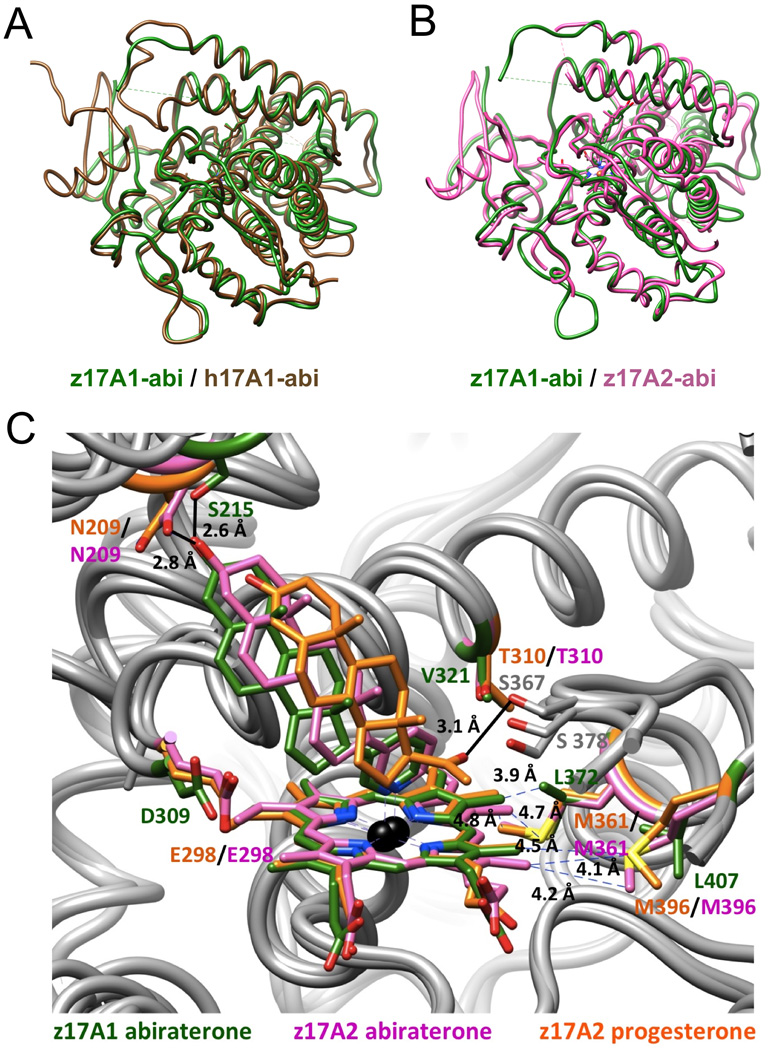
Similar tertiary structure folds of human P450 17A1 and zebrafish P450 17A1/A2 and subtle differences in the active sites of zebrafish P450 17A1 and 17A2 [103]. (A) Overlay of the abiraterone (abi) complexes of human P450 17A1 (PDB 3RUK; brown) [84] and zebrafish 17A1 (PDB 4R1Z; green). (B) Overlay of the abiraterone complexes of zebrafish P450 17A1 (green) and P450 17A2 (PDB 4R20; pink). (C) Comparison between the active site configurations of the zebrafish P450 17A1:abiraterone (green), 17A2:abiraterone (pink) and 17A2:progesterone (PDB 4R21; orange) complexes reveals five differences in the amino acid compositions of 17A1 (from human or zebrafish) and 17A2 (from zebrafish) in the vicinity of the heme moiety and near the ceiling of the active site. Color labels of residues match the corresponding structures.
