Abstract
An 80-year-old Japanese woman with wet cough and dyspnea was diagnosed with pneumonia at a clinic. Antibiotics did not improve her symptoms; therefore, she was referred to our hospital one month after symptom onset. Chest radiograph findings revealed complete collapse of the left lung. Bronchoscopy showed white mucus plug in the left main bronchus, which could not be removed. She was initially treated with bromhexine. Subsequently, culture results of the mucus plug specimen obtained during bronchoscopy yielded Schizophyllum commune. After three weeks, improvement of the collapsed lung was observed on chest radiograph.
Keywords: Schizophyllum commune, Allergic bronchopulmonary mycosis, Mucus plug, Expectorant
1. Introduction
Schizophyllum commune is a common species of fungus (mushroom) that colonizes diverse trees and rotting wood worldwide. Inhalation exposure to this environmental fungus is possible during usual activities of daily living. In 1994, Kamei reported the first case of allergic bronchopulmonary mycosis (ABPM) caused by S. commune infection [1]. Recently, an increasing number of cases of ABPM and mucoid impaction of the bronchi (MIB) secondary to S. commune infection has been reported, most of which were from Japan [2]. Some reports have indicated good response to treatment with antifungal drugs and/or corticosteroids; however, many patients develop resistance to such therapy [3], [4], [5], [6]. Therefore, there remain many unclear aspects on the treatment, as well the risk factors and pathogenesis, of ABPM and MIB caused by S. commune.
Herein, we report the first case of a favorable clinical effect of an expectorant agent against ABPM. This report may contribute to the improvement of treatment of ABPM caused by S. commune.
2. Case report
An 80-year-old Japanese female, with a 48-pack years smoking history, presented to a clinic complaining of wet cough and breathing difficulty. She had a past history of tuberculosis, but there was no history or symptoms suggestive of asthma. Chest radiograph revealed left lower lobe collapse (Fig. 1). She was hospitalized after being diagnosed with pneumonia and treated with antibiotics (ampicillin/sulbactam). However, the initial treatment was ineffective, and radiograph findings progressively worsened within weeks. Therefore, she was transferred to our hospital for evaluation and treatment at one month after symptom onset.
Fig. 1.
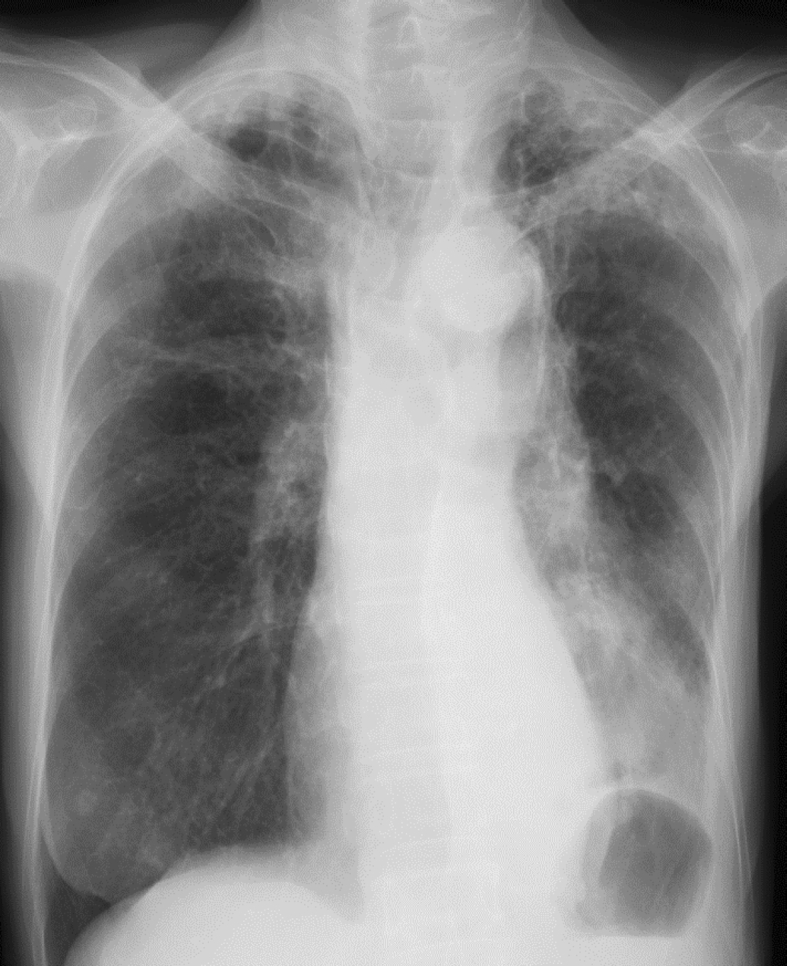
Chest radiograph on the first visit shows left lower lobe collapse.
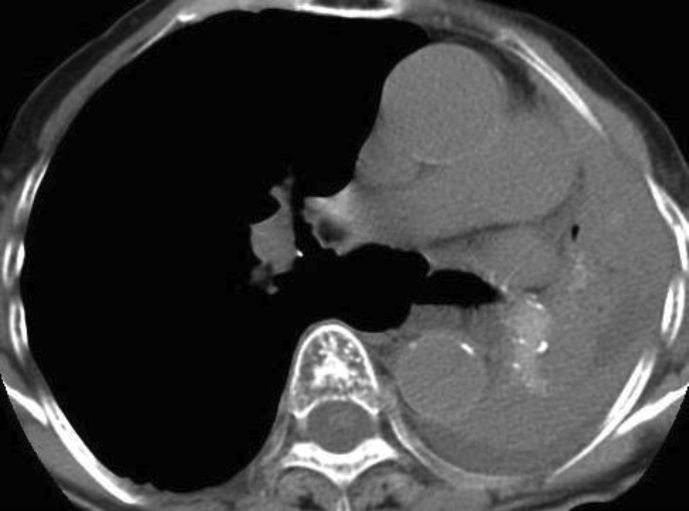
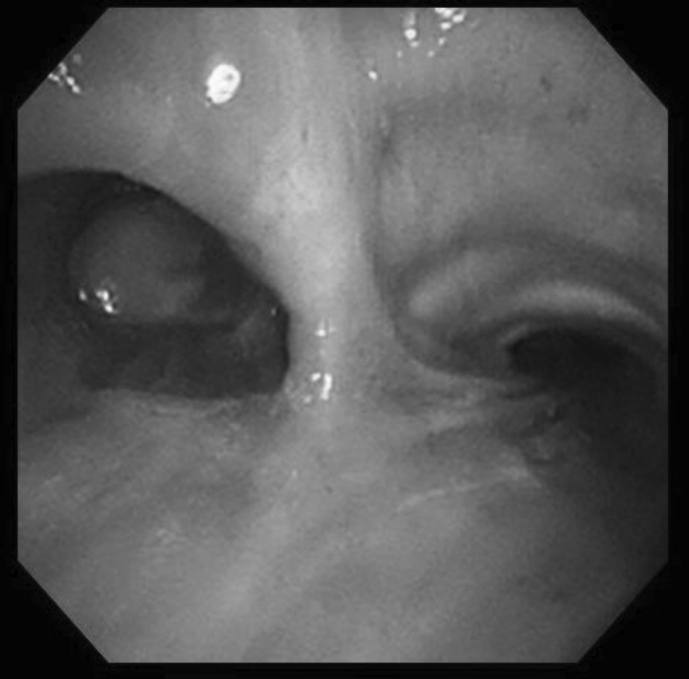
Upon examination, SpO2 was 89% at room air. Laboratory test showed elevated eosinophil count at 651/μL. Additionally, chest radiograph and computed tomography (CT) findings revealed complete collapse of the left lung with hyperattenuated mucoid (HAM) impaction (Fig. 2). Bronchoscopy showed a white, rubbery, thick mucus plug in the left main bronchus, which could not be removed by forceps or suction (Fig. 3). The findings on pathologic examination were eosinophilic infiltration without Charcot-Leyden crystals on Hematoxylin and Eosin stain and presence of fungal mycelium on Grocott stain. Culture of the small pieces of the mucus plug obtained during bronchoscopy yielded S. commune.
Fig. 2.
Chest computed tomography obtained upon admission shows complete collapse of the left lung with hyperattenuated mucoid impaction.
Fig. 3.
Bronchoscopy findings show white mucus plug in left main bronchus.
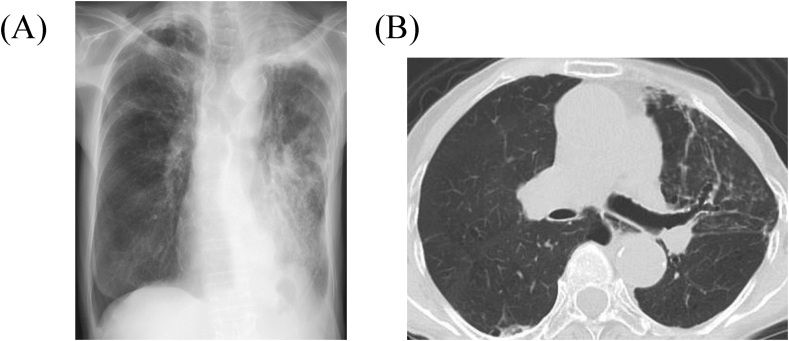
The patient was treated with an expectorant agent, bromhexine 8 mg three times daily, after the bronchoscopy. Three weeks later, chest radiographic findings revealed improvement of the collapsed left lung (Fig. 4A). Two months later, while she was completely recuperating from wet cough and breathing difficulty, SpO2 increased to 97% at room air and chest CT showed complete recovery of the collapsed lung, but with central bronchiectasis seen (Fig. 4B). Subsequently, she has returned to the clinic for follow-up and has continued bromhexine treatment successfully for more than 20 months. There were no adverse effects of bromhexine.
Fig. 4.
(A) Chest radiograph three weeks after initiation of bromhexine treatment shows improvement of the collapsed lung. (B) Chest computed tomography two months after initiation of bromhexine treatment shows central bronchiectasis and recovery of the collapsed lung.
3. Discussion
Antifungal drugs and/or corticosteroids are frequently the first choice of treatment for ABPM. According to the previously reported 18 cases of ABPM and MIB secondary to S. commune in Japan [1], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], patients were initially treated with antifungal drug (n = 6); systemic corticosteroid (n = 1); combination of antifungal drugs and systemic corticosteroids (n = 4), with the addition of inhaled corticosteroids (n = 3); bronchoscopic drainage (n = 1), with the addition of inhaled corticosteroids (n = 1); and combination of systemic and inhaled corticosteroids (n = 2). To the best of our knowledge, this was the first report of a favorable clinical effect of an expectorant agent alone in a patient with ABPM secondary to S. commune infection.
The efficacy of bromhexine is as follows: 1) promoting serous secretion, 2) dissolving content of acid glycoprotein fibers, and 3) increasing the ciliary movement. In addition, it has been reported that expectorant agents have anti-inflammatory effects [17], [18]. Therefore, a possible explanation for the efficacy of bromhexine in this case was that the activated ciliary movement and reduced inflammation facilitated removal of the dissolved mucoid impaction. Expectorants have less adverse effects and cost less compared with antifungal drugs and systemic corticosteroids. Based on these results, expectorant agents may be considered a routine add-on therapy to anti-fungal drugs and/or corticosteroids in patients with ABPM secondary to S. commune in order to obtain better response in the clinical setting.
There are no established diagnostic criteria for ABPM, especially ABPM secondary to S. commune infection. However, the diagnostic criteria for allergic bronchopulmonary aspergillosis (ABPA) are generally accepted for ABPM by replacing Aspergillus fumigatus with S. commune. In other words, the diagnostic criteria for ABPM caused by S. commune should include the clinical, serologic, pathologic, microbiologic, and imaging findings that are used for the diagnosis of ABPA. Katzenstein [19] and Bosken [20] have mentioned the importance of mucus plugs. Specifically, detecting an MIB that includes eosinophilic infiltration and fungus may be designated as ABPM. The present case was characterized by eosinophilic infiltration and the presence of S. commune in the mucus plugs. Therefore, we diagnosed as ABPM caused by S. commune. Goyal et al. revealed that HAM impaction on chest CT was seen in ABPA [21], which is characterized to be denser than skeletal muscle on CT. The possible reason for development of HAM is the presence of calcium salts and metals (e.g., ions of iron and manganese), hemorrhagic products, or desiccated mucus [21], [22], [23], [24]. HAM has been reported to be encountered in 18.7–30% of patients with ABPA [22], [25], [26], which is considered to be risk factor for relapse [25]. Uruga et al. first reported HAM caused by S. commune in 2010 [6]. Central bronchiectasis is also known to be a feature of ABPA on CT scan [27]. In addition, some reports revealed that central bronchiectasis was seen in ABPM caused by S. commune [2], [5], through a mechanism mediated by T helper 2-type immune responses and fungal proteases [28], [29], [30]. These CT scan findings were seen in our present case. Therefore, these characteristic CT findings may lead to a diagnosis of ABPM caused by S. commune.
The Rosenberg and Patterson criteria [27], in which S. commune replaces A. fumigatus, are most widely used for the diagnosis of ABPM caused by S. commune. The primary criteria include 1) episodic bronchial obstruction, 2) peripheral blood eosinophilia, 3) immediate skin reactivity to S. commune antigen, 4) precipitating antibodies against S. commune antigen, 5) elevated serum IgE concentration, 6) history of pulmonary infiltrates, and 7) central bronchiectasis. The secondary criteria include 1) S. commune in sputum, 2) history of expectoration of brown plugs, and 3) arthus reactivity to S. commune antigen. The presence of ≥6 primary criteria was reported by Agarwel et al. to have a 100% sensitivity and 100% specificity [31], whereas Ishiguro et al. reported a sensitivity of 57.1% and specificity of 100%. This difference in sensitivity of the criteria between the two studies may be accounted for by the varying characteristics of patients with asthma in the study by Ishiguro et al. In two other studies on patients with ABPM caused by S. commune, Kamei et al. reported that only 30% had bronchial asthma [32], whereas Glancy et al. reported that 74% had bronchial asthma [33]. Therefore, the present criteria may have low sensitivity for the diagnosis of ABPM caused by S. commune.
The diagnosis of ABPM was not made until culture of the mucus plug obtained during bronchoscopy revealed S. commune. Initially, our impression was a non-infectious mucus plug. For this reason, all diagnostic blood tests (serum IgE value, serum IgE antibody against S. commune, and serum precipitating or IgG antibody against S. commune), pulmonary function test, and treatment (antifungal drugs and/or corticosteroids) for ABPM were not performed. This was also the reason the patient was treated with bromhexine alone. Surprisingly, bromhexine was able to exert a therapeutic effect in this patient with ABPM secondary to S. commune infection by allowing drainage of the rubbery thick mucus plug that could not be removed by bronchoscopy.
In summary, we experienced a favorable clinical effect of an expectorant agent against ABPM due to S. commune. Expectorant agents, such as bromhexine, should be routinely used in the clinical setting as an add-on therapy that can work synergistically with antifungal drugs and/or corticosteroids in patients with ABPM caused by S. commune.
Conflict of interest
None.
Acknowledgements
The authors thank Katsuhiko Kamei of the Division of Clinical Research, Medical Mycology Research Center, Chiba University for the identification of S. commune.
References
- 1.Kamei K., Unno H., Nagao K., Kuriyama T., Nishimura K., Miyaji M. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Schizophyllum commune. Clin. Infect. Dis. 1994;18(3):305–309. doi: 10.1093/clinids/18.3.305. [DOI] [PubMed] [Google Scholar]
- 2.Chowdhary A., Randhawa H.S., Gaur S.N., Agarwal K., Kathuria S., Roy P., Klaassen C.H., Meis J.F. Schizophyllum commune as an emerging fungal pathogen: a review and report of two cases. Mycoses. 2013;56(1):1–10. doi: 10.1111/j.1439-0507.2012.02190.x. [DOI] [PubMed] [Google Scholar]
- 3.Amemiya Y., Shirai R., Tokimatsu I., Oka H., Iwata A., Otani S., Umeki K., Sakashita H., Ishii H., Gendo Y., Kishi K., Hiramatsu K., Kadota J. Allergic bronchopulmonary mycosis induced by Schizophyllum commune–case report and review of the literature. Nihon Kokyuki Gakkai Zasshi. 2009;47(8):692–697. [PubMed] [Google Scholar]
- 4.Ishiguro T., Takayanagi N., Harasaw K., Yoshii Y., Matsushita A., Yoneda K., Miyahara Y., Kagiyama N., Tokunaga D., Aoki F., Saito H., Ubukata M., Kurashima K., Yanagisawa T., Sugita Y., Kawabata Y., Kamei K. Mucoid impaction of the bronchi caused by Schizophyllum commune which developed after discontinuation of itraconazole administration. Nihon Kokyuki Gakkai Zasshi. 2009;47(4):296–303. [PubMed] [Google Scholar]
- 5.Masunaga A., Morimoto K., Ando T., Ikushima S., Takemura T., Oritsu M. Three cases of allergic bronchopulmonary mycosis due to Schizophyllum commune. Nihon Kokyuki Gakkai Zasshi. 2010;48(12):912–917. [PubMed] [Google Scholar]
- 6.Uruga H., Imafuku A., Hanada S., Takaya H., Miyamoto A., Sugimoto H., Morokawa N., Kurosaki A., Fujii T., Kishi K. A case of allergic bronchopulmonary mycosis caused by Schizophyllum commune presenting with hyperattenuated mucoid impaction. Nihon Kokyuki Gakkai Zasshi. 2010;48(10):749–754. [PubMed] [Google Scholar]
- 7.Tomita K., Hashizume I., Kasamatsu N., Nakamura A., Hanzawa S., Momiki S., Sasaki K., Okamoto K., Ozawa T., Kamei K. Allergic bronchopulmonary mycosis caused by Schizophyllum commune. Nihon Kyobu Shikkan Gakkai Zasshi. 1996;34(7):804–809. [PubMed] [Google Scholar]
- 8.Amitani R., Nishimura K., Niimi A., Kobayashi H., Nawada R., Murayama T., Taguchi H., Kuze F. Bronchial mucoid impaction due to the monokaryotic mycelium of Schizophyllum commune. Clin. Infect. Dis. 1996;22(1):146–148. doi: 10.1093/clinids/22.1.146. [DOI] [PubMed] [Google Scholar]
- 9.Yamashina S. Case of allergic bronchopulmonary mycosis caused by Schizophyllum commune. Jpn. J. Antibiot. 1997;50(1):51–53. discussion 54, 75-6. [PubMed] [Google Scholar]
- 10.Miyazaki Y., Sakashita H., Tanaka T., Kamei K., Nishimura K., Yoshizawa Y. Mucoid impaction caused by monokaryotic mycelium of Schizophyllum commune in association with bronchiectasis. Intern. Med. 2000;39(2):160–162. doi: 10.2169/internalmedicine.39.160. [DOI] [PubMed] [Google Scholar]
- 11.Itou Y., Sasaki S., Watanabe S., Kawamura T., Nakahara Y., Mochizuki Y., Kamei K. A case of mucoid impaction of bronchi (MIB) due to Schizophyllum commune. Nihon Kokyuki Gakkai Zasshi. 2001;39(4):266–270. [PubMed] [Google Scholar]
- 12.Yamasaki A., Nishimura K., Sano H., Tomita K., Chikumi H., Watanabe M., Hitsuda Y., Shimizu E. A case of allergic bronchopulmonary mycosis caused by Schizophyllum commune. Arerugi. 2002;51(5):439–442. [PubMed] [Google Scholar]
- 13.Kawano T., Matsuse H., Iida K., Kondo Y., Machida I., Saeki S., Tomari S., Miyazaki Y., Kohno S. Two cases of allergic bronchopulmonary mycosis caused by Schizophyllum commune in young asthmatic patients. Nihon Kokyuki Gakkai Zasshi. 2003;41(3):233–236. [PubMed] [Google Scholar]
- 14.Ishiguro T., Takayanagi N., Tokunaga D., Kurashima K., Matsushita A., Harasawa K., Yoneda K., Tsuchiya N., Yamaguchi S., Miyahara Y., Yano R., Saito H., Ubukata M., Yanagisawa T., Sugita Y., Kawabata Y. Pulmonary Schizophyllum commune infection developing mucoid impaction of the bronchi. Yale J. Biol. Med. 2007;80(3):105–111. [PMC free article] [PubMed] [Google Scholar]
- 15.Ishiguro T., Takayanagi N., Saito A., Akiyama K., Wakayama M., Shibuya K., Shimizu Y., Sugita Y., Kamei K. Allergic bronchopulmonary mycosis due to Schizophyllum commune and Aspergillus fumigatus. Nihon Kokyuki Gakkai Zasshi. 2011;49(8):612–618. [PubMed] [Google Scholar]
- 16.Kato F., Kasamatsu N., Kasai H., Nishimura R., Ogasawara T., Hashizume I. A case of mucoid impaction of bronchi caused by Schizophyllum commune. Jpn. Soc. Respir. Endosc. 2012;34(1):38–43. [Google Scholar]
- 17.Takeda K., Shiraishi Y., Matsubara S., Miyahara N., Matsuda H., Okamoto M., Joetham A., Gelfand E.W. Effects of combination therapy with montelukast and carbocysteine in allergen-induced airway hyperresponsiveness and airway inflammation. Br. J. Pharmacol. 2010;160(6):1399–1407. doi: 10.1111/j.1476-5381.2010.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maccio A., Madeddu C., Panzone F., Mantovani G. Carbocysteine: clinical experience and new perspectives in the treatment of chronic inflammatory diseases. Expert Opin. Pharmacother. 2009;10(4):693–703. doi: 10.1517/14656560902758343. [DOI] [PubMed] [Google Scholar]
- 19.Katzenstein A.L., Liebow A.A., Friedman P.J. Bronchocentric granulomatosis, mucoid impaction, and hypersensitivity reactions to fungi. Am. Rev. Respir. Dis. 1975;111(4):497–537. doi: 10.1164/arrd.1975.111.4.497. [DOI] [PubMed] [Google Scholar]
- 20.Bosken C.H., Myers J.L., Greenberger P.A., Katzenstein A.L. Pathologic features of allergic bronchopulmonary aspergillosis. Am. J. Surg. Pathol. 1988;12(3):216–222. doi: 10.1097/00000478-198803000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Goyal R., White C.S., Templeton P.A., Britt E.J., Rubin L.J. High attenuation mucous plugs in allergic bronchopulmonary aspergillosis: CT appearance. J. Comput. Assist. Tomogr. 1992;16(4):649–650. [PubMed] [Google Scholar]
- 22.Logan P.M., Muller N.L. High-attenuation mucous plugging in allergic bronchopulmonary aspergillosis. Can. Assoc. Radiol. J. 1996;47(5):374–377. [PubMed] [Google Scholar]
- 23.Kopp W., Fotter R., Steiner H., Beaufort F., Stammberger H. Aspergillosis of the paranasal sinuses. Radiology. 1985;156(3):715–716. doi: 10.1148/radiology.156.3.4023231. [DOI] [PubMed] [Google Scholar]
- 24.Dillon W.P., Som P.M., Fullerton G.D. Hypointense MR signal in chronically inspissated sinonasal secretions. Radiology. 1990;174(1):73–78. doi: 10.1148/radiology.174.1.2294574. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R., Gupta D., Aggarwal A.N., Saxena A.K., Chakrabarti A., Jindal S.K. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: an analysis of 155 patients. Chest. 2007;132(4):1183–1190. doi: 10.1378/chest.07-0808. [DOI] [PubMed] [Google Scholar]
- 26.Franquet T., Muller N.L., Gimenez A., Guembe P., de La Torre J., Bague S. Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics. 2001;21(4):825–837. doi: 10.1148/radiographics.21.4.g01jl03825. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg M., Patterson R., Mintzer R., Cooper B.J., Roberts M., Harris K.E. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann. Intern. Med. 1977;86(4):405–414. doi: 10.7326/0003-4819-86-4-405. [DOI] [PubMed] [Google Scholar]
- 28.Kauffman H.F. Immunopathogenesis of allergic bronchopulmonary aspergillosis and airway remodeling. Front. Biosci. 2003;8:e190–e196. doi: 10.2741/990. [DOI] [PubMed] [Google Scholar]
- 29.Knutsen A.P., Bellone C., Kauffman H. Immunopathogenesis of allergic bronchopulmonary aspergillosis in cystic fibrosis. J. Cyst. Fibros. 2002;1(2):76–89. doi: 10.1016/s1569-1993(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 30.Chauhan B., Knutsen A., Hutcheson P.S., Slavin R.G., Bellone C.J. T cell subsets, epitope mapping, and HLA-restriction in patients with allergic bronchopulmonary aspergillosis. J. Clin. Investig. 1996;97(10):2324–2331. doi: 10.1172/JCI118675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal R., Maskey D., Aggarwal A.N., Saikia B., Garg M., Gupta D., Chakrabarti A. Diagnostic performance of various tests and criteria employed in allergic bronchopulmonary aspergillosis: a latent class analysis. PLoS One. 2013;8(4):e61105. doi: 10.1371/journal.pone.0061105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamei K., Unno H., Ito J., Nishimura K., Miyaji M. Analysis of the cases in which Schizophyllum commune was isolated. Jpn. J. Med. Mycol. 1999;40:175–181. doi: 10.3314/jjmm.40.175. [DOI] [PubMed] [Google Scholar]
- 33.Glancy J.J., Elder J.L., McAleer R. Allergic bronchopulmonary fungal disease without clinical asthma. Thorax. 1981;36(5):345–349. doi: 10.1136/thx.36.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]