Abstract
This work describes that cytotoxicity of lead chloride and lead acetate to in vitro cultured lymphocytes from human umbilical cord blood, using four monitoring methods namely, trypan blue staining, acridine orange/ethidium bromide staining, 3-[4,5-dimethylthiazol-2-yl] 2,5-diphenyl tetrazolium bromide (MTT) and neutral red uptake assays; lead genotoxicity to lymphocytes was monitored by comet assay. The MIC value in each method was invariably 300 mg/L for PbCl2. Lethal concentration25 (LC25) values were almost in an agreeable range: 691.83 to 831.76 mg/L; LC50 values in each method were almost in the range: 1174.9 to 1348.9 mg/L; LC100 values were in the range: 3000 to 3300 mg/L, for lead chloride. Similarly, The MIC value in each method were invariably 150 mg/L; LC25 values were almost in the range: 295.12 to 371.53 mg/L; LC50 values were in the range: 501.18 to 588.84 mg/L; LC100 value was 1500 mg/L in all assays, for lead acetate. The comet assay also indicated that the LC100 values were 3300 mg/L lead chloride and 1500 mg/L lead acetate. Thus, both cytotoxicity and genotoxicity were recorded at 3300 mg/L lead chloride and 1500 mg/L lead acetate with lymphocytes.
Keywords: cytotoxicity, genotoxicity, lead chloride, lead acetate, AO/EB staining, MTT assay, comet assay
Introduction
Without any role in metabolism, lead or Plumbum (Pb, Group 2b) is a toxic heavy metal. Effluents from industries dealing with paper, food canning, battery and cosmetics as well as mining industry, oil refineries and coal mine establishments mainly add Pb compounds to the total environment. Of all Pb compounds produced in the United States, for example, a 4% (80,000 tons/year) approximately is made into bullets; consequently, 58,300 tons/year Pb approximately from shot and munitions get deposited onto landscape through shooting activities (USEPA, 2001). Pb is present in environments in soluble, PbCl2, CH3COOPb (lead acetate) and PbCO3, as well as insoluble PbS, Pb3(PO4)2, PbO and PbSO4 forms, apart from spillage as ore powders from establishments dealing with Pb-minerals, hydrocerussite, cerussite and massicot. Moreover, leaching of water soluble Pb compounds in soil in the rainy season causes the Pb-sorption by biota (Sharma et al., 2010, Moussa et al., 2008, Campbell et al., 2004).
From studies on lead toxicity with Wistar rats by injecting lead acetate solution at 15 mg/kg body weight, a significant decrease in the level of superoxide dismutase activity and increase in plasma bilirubin level were recorded (Berrahal et al., 2007, Rogers et al., 2003). A report in rat system showed that the ingestion of Pb induced stimulation in glutamic-pyruvic transaminase and glutamic-oxalacetic transaminase activity; the cholinesterase activity was inhibited, while hyperglycemia was induced in lead acetate toxicity; in blood, metallic Pb reduced hemoglobin contents and red blood cell (RBC) count as well as the plasma levels of T3, T4 and white blood cell count had decreased (Ewis et al., 2012). In Wistar rats the relative retention of lead acetate by the issues was determined as follows: lungs>spleen>stomach>kidney> blood>heart in males and in females as spleen>stomach> heart>kidney>blood>lungs in the ip-route, and those were kidney>lungs>stomach>blood>heart>spleen, in males and kidney>lungs>stomach>blood>heart>spleen in females in the oral route (Jarrar & Taib, 2012). A low level exposure to lead acetate reported to cause adverse effects on neurons of zebrafish embryos (Zhang et al., 2012).
A variety of toxic effects caused by Pb compounds had been identified during gestation and lactation in animals and humans (Bunn et al., 2001). Lead exposure was demonstrated to occur both through respiratory and gastro-intestinal tracts (Park et al., 2008). About a 60% of ingested lead was reported to be absorbed by empty stomach, but along with food a lesser amount (15-20%) was absorbed. Obviously, blood is the inadvertent route of the metal mobility to brain, liver, bone marrow and testis. Furthermore, child-neuro development and adult brain cells were recorded to be affected by lead toxicity (Bellinger, 2008). It was reported that because of the exposure of the mother to pollutants, the fetus in womb was affected by lead poisoning with the eventual impairment of the natural development of immune system in the postnatal life (Bishoyi & Sengupta, 2006). Ultimately, Pb gets stored in bone inducing decrease in bone mineral density (Lee et al., 2010). Monitoring of levels of renal thioredoxin reductase-1 activity in rats exposed to 25 mg/kg lead acetate was recorded to induce renal damage (Conterato et al., 2014). And it was demonstrated that lead acetate at 1 and 1.5 g/L caused male sterility in rats in a dose dependent manner (Wang et al., 2013). Increase of low density lipoprotein cholesterol, atherogenetic index and coronary heart disease index levels with exposure to lead acetate in broiler chicken were, 45.24%, 100%, 16.66% compared to the control group, in a study (Karimi et al., 2013).
Apart from gastro-intestinal disturbances in humans, lead toxicity causes restlessness, irritability, headache, hepatic and renal damage, hypertension, hallucination and encephalopathy; in blood basophilic stippling and decreased hemoglobin synthesis occur due to Pb (Anonymous, 2007). Age related impairments in behavioral functions had been linked to lead poisoning that caused changes in neurotransmitter levels in brain tissue (Chand et al., 2014). Lead induced biochemical and structural changes occur in liver causing cataclysmic alterations by oxidative stress, apoptosis and mitogen activated protein kinase (Mujaibel & Kilarkaje, 2013, Samaraghandian et al., 2013). Particularly, oxidative stress had been reported to be one of several important possible mechanisms in lead toxicity (Shalan et al., 2005) with the generation of hydroxyl radical, hydrogen peroxide, superoxide anion and lipid peroxidase, etc. (Soltaninejad et al., 2003).
A single report on the use of human umbilical cord mesenchymal stem cells had been recorded monitoring lead trioxide toxicity at <10 mM; lead acetate too had toxic effect on the self-renewal, multipotent differentiation potential and hematopoiesis-promoting function of mesenchymal stem cells from umbilical cord blood (UCB) (Sun et al., 2012). Pb had been reported to have toxicity to hematopoietic system by interfering with hemoglobin synthesis and erythrocyte morphology. In this study, cell count, 3-[4,5-dimethylthiazol-2-yl] 2,5-diphenyl tetrazolium bromide (MTT) assay, apoptosis assay, osteogenic differentiations were recorded to be affected by lead trioxide. Alkaline phosphatase enzyme was lower than control group (Sun et al., 2012). DNA damage by lead acetate exposure was reported in the rabbit model at 15 mg/kg; infraction of the kidneys and intranuclear specific inclusion bodies in liver and kidney were detected (Ahmed et al., 2012).
This work describes toxicity of PbCl2 and lead acetate to in vitro cultured lymphocytes from human UCB. Cyto-toxicity was monitored using trypan blue (TB) and acridine orange/ethidium bromide (AO/EB) staining, MTT and neutral red uptake (NRU) assays; genotoxicity was monitored by comet assay. Cord blood being a waste blood, its use in experiment in ‘predictive toxicity’, such as herein, should not warrant ethical issues. Predictive toxicology is an offshoot of pharmacology describing concepts related to toxic effects of newer chemicals and prospective drugs in model systems to predict health-risk assessments, before institutional recommendation as a drug and other uses. The underlying method could well be adopted for environmental toxicants, for planning well-being of human health. Differentiated cells obtained from a mass of lymphocytes could be able to mimic some actions related to chemical kinetics operative naturally in metabolism as a response to the chemical. Thus, this would help examine the probable role of some toxic chemicals in human system. Lymphocytes help the defense mechanism of the body against infectious agents, due to their ability to distinguish the body’s own cells from the foreign ones.
Materials and methods
Collection of lymphocytes
With an aliquot of 100 or 250 μL 1,000 IU heparin (HiMedia, Mumbai), in a sterile 15 or 50 mL size falcon tube (Tarson, Kolkata), the UCB sample was collected according to volume, immediately after the delivery of an infant and was stored at 4 °C till use. Lymphocytes were isolated immediately or within at the best 24 hrs after the collection of UCB. For the isolation of lymphocytes, the collected UCB sample was diluted with phosphate buffered saline (PBS) solution in 1:1 proportion. The mixture was loaded carefully into a centrifuge tube for over-layering on Histopaque (Sigma, Mumbai) for the separation of lymphocytes, which was one-third the total volume of the mixture. The mixture was centrifuged at 1,800 rpm for 25 min at 22–24 °C; and four heavy to light layers, RBC, Histopaque, buffy coat and plasma were seen (Figure 1). Mononuclear cells in the buffy coat layer were taken out carefully from the tube. To the separated cells, another aliquot of PBS in the 1:1 ratio was added, mixed gently and re-centrifuged at 2,000 rpm for 5 min. The pellet of lymphocytes was taken for culturing and subsequently cell counts were done using a haemocytometer.
Figure 1.
Density gradient centrifugation of human umbilical cord blood.
Growth of lymphocytes
After separation, UCB-derived lymphocytes were diluted to the density of 1×106 cells/mL with a required volume of Dulbecco’s modified Eagle’s medium (DME medium-lowglucose, HiMedia, Mumbai), and were loaded into a 6-well culture plate (Tarson); DME medium contained 15% fetal bovine serum (Sigma), 1% penicillin-streptomycin and 1% sodium pyruvate, along with graded concentrations of PbCl2 or lead acetate solution for growth. The stock solution was prepared by dissolving 1000 mg of PbCl2 or lead acetate (Sigma) in aliquots of 100 mL of triple distilled water for the final concentration of 10,000 mg/L, and the stock solution was stored at 4ºC, for further use. The volume of 2 mL in total mixture was maintained in each well of the culture plate; the cells were incubated with different concentrations of PbCl2 (0, 300, 600, 900, 1200, 1500, 1800, 2100, 2400, 2700, 3000 and 3300 mg/L), or lead acetate (0, 150, 300, 450, 600, 750, 900, 1050, 1200, 1350 and 1500 mg/L) in an incubator at 37 °C in 5% atmospheric CO2 concentration for 24 hrs for growth (Figure 2).
Figure 2.
Photomicrograph of growing lymphocytes; magnification, ×400.
Assessment of cyto- and genotoxicity
The viability of lymphocytes grown in the presence of PbCl2 and lead acetate was assessed using two staining procedures, with TB and AO/EB, using a phase-contrast (Magnus, New Delhi) and fluorescent microscope (Magnus), respectively. MTT assay and NRU assay were also carried out as the standard procedures for monitoring the live cell density. Comet assay was done for assessing Pb-induced nuclear damages.
TB staining
TB solution was prepared in PBS at the concentration of 0.4 g/mL. For the study of cell viability to the in vitro grown mass of lymphocytes, TB solution was added at the 1:1 ratio; the mixture was kept in an incubator for 2 min at 37 °C and cells were observed under the phase-contrast microscope at the 400× magnification. The live cells remained unstained, whereas the nuclei of the dead cells were blue in appearance, as TB enters dead cells and stains the nuclei to blue, as it is a membrane permeable dye.
AO/EB staining
The AO/EB solution was prepared in PBS at the concentration of 100 μg/mL and is applied to cultured lymphocytes after a 24 hrs of incubation in presence of graded concentrations of any of the two Pb compounds. When observed under the fluorescent microscope at 400×, green colour indicated live cells, whereas cells with orange and red colour were apoptotic and necrotic cells, respectively (Figure 3). AO is taken by both live and dead cells and emits green fluorescence. EB is only taken up by cells when the integrity cytoplasmic membrane is lost and stains nucleus orange. Hence live cells, apoptotic cells and necrotic cells were green, orange and red in appearance respectively. Toxicity values were obtained with different concentrations of PbCl2 and lead acetate (Figure 4). Percent lethality (PL) values of the third repeated experiment were transformed to probit values by Finney’s method, which were potted against corresponding log10 values of PbCl2 and lead acetate concentrations, as exemplified before (Rath et al., 2011). Probits of observed lethality percentage values were from statistical tables of probit transformation.
Figure 3.
Probits of percentage lethality values plotted against log10 concentrations of PbCl2 and CH3COOPb (lead acetate) in the toxicity study of lymphocytes by MTT assay; each line is fitted by eye; three pairs of log10 concentration values were determined taking probit points, 4.3255 (LC25), 5.0000 (LC50) and 5.6745 (LC75).
Figure 4.
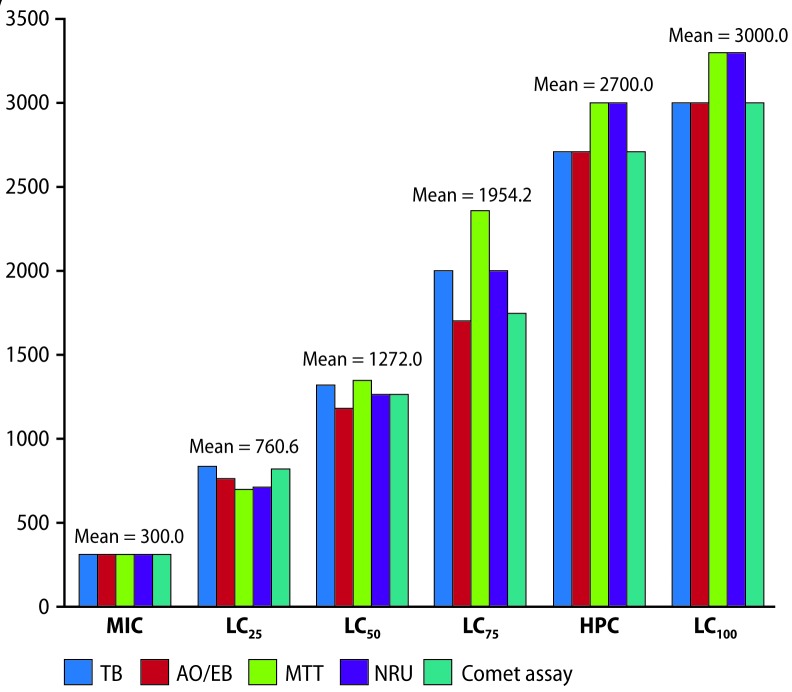
Histogram of MIC, LC25, LC50, LC75, HPC and LC100 values corresponding to TB, AO/EB, MTT, NRU and comet assay methods (see text for abbreviations) after the lymphocytes treated with PbCl2. M= mean value (mg/L).
MTT assay
The MTT stock solution was prepared at the concentration of 5 mg/mL in PBS. After 24 hrs of toxin treatment in 6-well culture plate, 80 μL of MTT stock solution was added to each well to study the toxicity effect. The plate was kept in an incubator (37 °C, 5% CO2) for 4 hrs and it was found that media containing cells and toxin got converted to blue colour after incubation with MTT. The mixture was gently centrifuged at 1,000 rpm for 10 min at 22 °C. The supernatant was removed and the pellet was dissolved in 1 mL aliquot of 100% dimethyl sulfoxide (DMSO) and the suspension was kept in an incubator for 1 hr. Optical density570 of the suspension with the purple colour was measured with a spectrophotometer. Percentage of cell density was calculated as follows:
MTT in DMSO solution was taken as the blank. Probits of observed lethality percentage values and log10 concentration values of chemical were used for analysis of toxicity.
NRU assay
Neutral red solution was prepared in serum-free DME medium at the concentration of 100 μg/mL. After 24 hrs growth of lymphocytes in the presence of a toxin in a 6-well culture plate, an aliquot of 200 μL of neutral red dye solution was added to each well. The plate was kept in an incubator (37 °C, 5% CO2) for 3 hrs. Indeed, lysosomes of viable cells absorb the dye and the dead cells remain unstained. Thereafter, an aliquot of 1 mL of separately prepared neutral red desorption solution (1% acetic acid, 50% ethanol and 49% distilled water) was added to each well for removal the dye from lysosomes of live cells. Optical density540 of the washout with a spectrophotometer was measured that assessed live cell density whose percentage values were calculated.
Comet assay
Single cell gel electrophoresis was carried out to study DNA damage of the cells treated with different concentrations of PbCl2 and lead acetate. Lymphocytes cultured with different concentrations of a Pb-chemical were harvested and used in the alkaline comet assay technique. Slides were coated with 1% agarose and were allowed for air drying. Pellets of lymphocytes, obtained by centrifugation of cultured cells were washed with PBS, and the pellet was mixed with three times the cell volume of the pellet with low melting point agarose (LMPA) 1% in sol state. The mixture of cells and LMPA sol was placed over the agarose coated slide that was kept at 4 °C for 10 min until the slide got dry. The dried slides were submerged into a pre-cooled lysing solution of the mixture of 0.4 M Tris HCl, 0.8 M dithiothreiotl (DTT) and 1% sodium dodecyl sulphate (SDS) of pH 7.5 and the mixture was kept at 4 °C in dark for about 30 min. The slides were subsequently removed and placed in another lysing solution with 0.4 M Tris HCl, 2 M NaCl, 1% SDS, 0.05 M EDTA at pH 7.5 for 30 min. The slides were placed in tris borate EDTA (TBE) buffer, which contained 89 mM Tris, 89 mM Boric acid and 2.5 mM EDTA at pH 8.3 for 10 min. The slides were transferred to a horizontal gel electrophoretic chamber with TBE buffer. Electrophoresis was carried out at 20 V and 12 mA for 12.5 min. After the electrophoresis, the slides were washed with 0.9% NaCl for 2.5 min, and the second electrophoresis was carried out at 20 V, 12 mA for 4 min in alkaline solution, which contained, 0.03 M NaOH and 1 M NaCl. The slides were placed in a neutralizing solution with 0.4 M Tris HCl for 5 min, and again the slides were washed in TBE buffer. After 5 min, the slides were stained with EB solution and were observed under the fluorescence microscope at 400×, for scoring the comets. Probits of observed lethality percentage values calculated from percent values of observed comets due to PbCl2 or lead acetate treatment; probits analyses of toxicity data were done.
Results
Percent lethality (PL) values recorded from data sets of tests with TB, AO/EB, MTT and NRU assays were transformed to their probits were used to construct respective plots against Pb concentrations in log scale (Figure 3); plots were used for extrapolation to compute individual lethal concentration (LC) values, LC25, LC50 and LC75 in each method. The individual minimum inhibitory concentration (MIC), highest permissive concentration (HPC) and LC100 values were noted directly from experiments. Data from all the toxicity monitoring experiments were summarized for probit analysis of toxicity and all LC values were cited (Tables 1–4).
Table 1.
Probit transformations of percent lethality values during PbCl2 toxicity to human lymphocytes growing in DME medium, assessed by four methods, TB, AO/EB staining, MTT assay, NRU assay and comet assay.
| PbCl2(ppm) | Log10 concen-tration of PbCl2 | PL of cells by TB staining | Probits of TB staining | PL of cells by AO/EB staining | Probits of AO/EB staining | PL of cells by MTT assay | Probits of MTT assay | PL of cells by NRU assay | Probits of NRU assay | DFI (%) | Probits of DFI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 300 | 2.47 | 8.3 | 3.6 | 12 | 3.8 | 8.6 | 3.6 | 4.5 | 3.3 | 9 | 3.6 |
| 600 | 2.77 | 19.8 | 4.2 | 25 | 4.3 | 15.8 | 3.9 | 16.9 | 4.0 | 21 | 4.2 |
| 900 | 2.95 | 24.0 | 4.3 | 36 | 4.6 | 23.2 | 4.2 | 28.1 | 4.4 | 35 | 4.6 |
| 1200 | 3.07 | 36.6 | 4.6 | 42 | 4.7 | 41.3 | 4.7 | 40.5 | 4.7 | 41 | 4.7 |
| 1500 | 3.17 | 48.4 | 5.9 | 54 | 5.1 | 49.4 | 4.9 | 51.7 | 5.0 | 55 | 5.1 |
| 1800 | 3.25 | 60.3 | 5.2 | 70 | 5.5 | 59.5 | 5.2 | 64.1 | 5.3 | 69 | 5.4 |
| 2100 | 3.32 | 73.5 | 5.6 | 82 | 5.9 | 68.1 | 5.4 | 67.5 | 5.4 | 83 | 5.9 |
| 2400 | 3.38 | 85.0 | 6.0 | 89 | 6.2 | 86.3 | 6.1 | 79.8 | 5.8 | 91 | 6.3 |
| 2700 | 3.43 | 92.2 | 6.4 | 94 | 6.5 | 96.1 | 6.7 | 91.1 | 6.3 | 96 | 6.7 |
| 3000 | 3.47 | 100 | - | 100 | - | 98.6 | 7.1 | 93.3 | 6.4 | 100 | - |
| 3300 | 3.51 | - | - | - | - | 100 | - | 98.9 | 72 | - | - |
Note: TB, Trypan blue; AO/EB, Acridine orange/ethidium bromide; DMEM, Dulbecco's modified Eagle's medium; MTT, 3-[4, 5- dimethylthiazol-2-yl] 2,5-diphenyl tetrazolium bromide; NRU, Neutral red uptake assay; PL, Percent lethality; DFI, DNA fragmentation index; -, Not applicable. Experiments were repeated thrice and the last data set was presented.
Table 4.
Toxicity values of CH3COOPb to human lymphocytes obtained by experimentation and the probit computation.
| Toxicity values (mg/L) | ||||||
|---|---|---|---|---|---|---|
| Assay methods | MIC* | LC25 | LC50 | LC75 | HPC* | LC100* |
| TB staining | 150 | 295.12 | 588.84 | 851.13 | 1350 | 1500 |
| AO/EB staining | 150 | 338.84 | 512.86 | 954.99 | 1350 | 1500 |
| MTT assay | 150 | 371.53 | 524.81 | 707.94 | 1350 | 1500 |
| NRU assay | 150 | 323.59 | 501.18 | 707.94 | 1350 | 1500 |
| Comet assay | 150 | 331.13 | 512.86 | 758.57 | 1350 | 1500 |
Note: *From experiments
TB staining
TB staining indicated that cell counts decreased gradually after growing lymphocytes with PbCl2 at graded levels of 300 to 3000 mg/L, whereas similar results of decreased pattern of live cell count from 150 to 1500 mg/L level with lead acetate. The MIC value was recorded as 300 mg/L PbCl2, HPC and LC100 values were 2700 and 3000 mg/L PbCl2, respectively; whereas MIC, HPC and LC100 values were 150, 1350 and 1500 mg/L lead acetate, respectively. From the probit plot, it was ascertained that for values of LC25, LC50 and LC75, for probit values, 4.33, 5.00 and 5.67, respectively, the corresponding log10 concentration values were 2.92, 3.12 and 3.30, respectively for PbCl2 (Figure 4, Table 2); whereas for lead acetate, these values were 2.47, 2.77 and 2.93, respectively. Antilog values of these log10 concentration values were the following LC values, 831.76 (LC25), 1318.25 (LC50), 1995.26 (LC75) mg/L PbCl2 and 295.12 (LC25), 588.84 (LC50), 851.13 (LC75) mg/L lead acetate (Figure 5, Table 4).
Table 2.
Toxicity values of PbCl2 to human lymphocytes obtained by experimentation and the probit computation.
| Toxicity values (mg/L) | ||||||
|---|---|---|---|---|---|---|
| Assay methods | MIC* | LC25 | LC50 | LC75 | HPC* | LC100* |
| TB staining | 300 | 831.76 | 1318.2 | 1995.3 | 2700 | 3000 |
| AO/EB staining | 300 | 758.57 | 1174.9 | 1698.2 | 2700 | 3000 |
| MTT assay | 300 | 691.83 | 1348.9 | 2344.2 | 3000 | 3300 |
| NRU assay | 300 | 707.94 | 1258.9 | 1995.3 | 3000 | 3300 |
| Comet assay | 300 | 812.83 | 1258.9 | 1737.8 | 2700 | 3000 |
Note: *From experiments
Figure 5.
Histogram of MIC, LC25, LC50, LC75, HPC and LC100 values corresponding to TB, AO/EB, MTT, NRU and comet assay methods (see text for abbreviations) after the cells treated with lead acetate. M= mean value (mg/L).
AO/EB staining
Treatment of cells with different concentrations of PbCl2 for 24 hr resulted in a decreasing pattern of living cell counts (Figure 6). The number of dead cells gradually increased from the level of 300 to 2700 mg/L level, and it was found that there were no cells at the 3000 mg/L level. In case of lead acetate, the decreasing pattern of live cell count started from 150 to 1350 mg/L level, whereas there were no cells at 1500 mg/L. Experimentally, the lethality was seen at 300 mg/L, which was recorded as the MIC value, the HPC was 2700 mg/L, and the LC100 value was 3000 mg/L for PbCl2; and for lead acetate the MIC, HPC and LC100 values were 150, 1350 and 1500 mg/L, respectively. From the plot, for PbCl2 it was ascertained that for values of LC25, LC50, and LC75, the corresponding log10 concentration values were 2.88, 3.07 and 3.23, respectively (Figure 4, Table 2); and for lead acetate the values were 2.53, 2.71, 2.98, respectively. Antilog values of these log10 concentration values are 758.57 (LC25), 1174.89 (LC50) and 1698.24 (LC75) mg/L, which were the computed LC values of PbCl2, and for lead acetate the computed LC values were 338.84 (LC25), 512.86 (LC50) and 954.99 (LC75) mg/L (Figure 5, Table 4).
Figure 6.
AO/EB staining. Control cells (A); Cells after treated with 1500 mg/L PbCl2(B); Cells after treated with 1500 mg/L lead acetate (C).
MTT assay
The cell density at OD570 gradually decreased from the level of 300 mg/L to 3000 mg/L and it was found that there were no cells at the 3300 mg/L PbCl2 level. Experimentally, the MIC value was 300 mg/L, whereas the HPC was 3000 mg/L, and the LC100 value was 3300 mg/L PbCl2. With lead acetate, the MIC was 150, whereas the HPC and LC100 values were 1350 and 1500 mg/L. Extrapolated log10 values of PbCl2 from the plot were 2.84, 3.13 and 3.37, respectively as LC25, LC50, and LC75 log10 values (Figure 4, Table 2); whereas similar values for lead acetate were 2.57, 2.72, 2.85, respectively. Thus, these log10 concentration values generated LC values: 691.83 (LC25), 1348.96 (LC50) and 2344.22 mg/L (LC75) for PbCl2, and 371.53 (LC25), 524.81 (LC50), 707.94 mg/L (LC75) for lead acetate (Figure 5, Table 4).
NRU assay
Cell density, monitored at OD540 gradually decreased from the level of 300 to 3300 mg/L PbCl2. Experimentally, the MIC value was 300 mg/L; whereas the HPC was 3000 and the LC100 was 3300 mg/L PbCl2. Log10 values of PbCl2 concentrations extrapolated from the probit plot yielded log10 values 2.85, 3.10 and 3.30, for values of LC25, LC50, and LC75, respectively (Figure 4, Table 2); and for lead acetate these values were 2.51, 2.70 and 2.85, respectively. Further, these log10 concentration values generated LC values: 707.94 (LC25), 1258.92 (LC50) and 1995.26 mg/L (LC75) PbCl2, and 323.59 (LC25), 501.18 (LC50), 707.94 mg/L (LC75) lead acetate (Figure 5, Table 4).
Comet assay
It was observed that, comet tail was found increasing in cells treated with 300 mg/L to 3000 mg/L PbCl2 and 150 to 1500 mg/L for lead acetate (Figure 7); DNA fragmentation index (DFI) too increased with increasing gradations of both lead chemicals (Tables 1 and 3). From probit analysis, it was found that LC25, LC50 and LC75 values were 812.83, 1258.9 and 1737.8 mg/L PbCl2, respectively (Figure 4, Table 2); whereas similar values were 331.13, 512.86, 758.57, respectively for lead acetate. The MIC value as 300, the HPC value as 2700 and the LC100 value as 3000 mg/L PbCl2, were recorded experimentally while, similar values were 150 (MIC) and 1350 (HPC), 1500 (LC100) mg/L for lead acetate (Figure 5, Table 4).
Figure 7.
Comet assay. Control cells (A); Cells after treated with 1500 mg/L PbCl2 (B); Cells after treated with 1500 mg/L lead acetate (C).
Table 3.
Probit transformations of percent lethality values during CH3COOPb toxicity to human lymphocytes growing in DMEM, assessed by four methods, TB, AO/EB staining, MTT assay, NRU assay and comet assay.
| Conc. of CH3COOPb (mg/L) | Log10 concentration of CH3COOPb | PL of cells by TB staining | Probits of TB staining | PL of cells by AO/EB staining | Probits of AO/EB staining | PL of cells by MTT assay | Probits of MTT assay | PL of cells by NRU assay | Probits of NRU assay | DFI (%) | Probits of DFI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | - | 0 | - | 0 | - | 0 | - | - | - | - | - |
| 150 | 2.17 | 16 | 4.0 | 12 | 3.8 | 13.6 | 3.9 | 12.1 | 3.8 | 11 | 3.7 |
| 300 | 2.47 | 24 | 4.3 | 21 | 4.2 | 20.2 | 4.2 | 20.3 | 4.2 | 20 | 4.1 |
| 450 | 2.65 | 39 | 4.7 | 33 | 4.6 | 34.6 | 4.6 | 32.3 | 4.5 | 32 | 4.5 |
| 600 | 2.77 | 47 | 4.9 | 46 | 4,9 | 39.1 | 4.7 | 40.5 | 4.7 | 47 | 4.9 |
| 750 | 2.87 | 56 | 5.2 | 52 | 5.1 | 59.5 | 5.2 | 51.9 | 5.0 | 53 | 5.1 |
| 900 | 2.95 | 67 | 5.4 | 61 | 5.3 | 77.1 | 5.7 | 62.3 | 5.3 | 64 | 5.3 |
| 1050 | 3.02 | 79 | 5.8 | 74 | 5.6 | 85.3 | 6.0 | 74.9 | 5.7 | 76 | 5.7 |
| 1200 | 3.07 | 86 | 6.1 | 89 | 6.2 | 94.4 | 6.6 | 84.7 | 6.0 | 88 | 6.1 |
| 1350 | 3.13 | 93 | 6.5 | 96 | 6.7 | 97.2 | 6.9 | 94.0 | 6.5 | 94 | 6.5 |
| 1500 | 3.17 | 100 | - | 100 | - | 98.3 | 7.1 | 97.9 | 7.0 | 100 | - |
Note: TB, Trypan blue; AO/EB, Acridine orange/ethidium bromide; DME medium, Dulbecco’s modified Eagle’s medium; MTT, 3-[4, 5- dimethylthiazol-2-yl] 2,5-diphenyl tetrazolium bromide; NRU, Neutral red uptake assay; PL, Percent lethality; DFI, DNA fragmentation index; –, Not applicable. Experiments were repeated thrice and the last data set was presented.
Discussion
This work demonstrated that four methods of monitoring cytotoxicity of lymphocytes, namely TB staining, AO/EB staining, MTT assay and NRU assay could give dependable results. The MIC value in each method were invariably 300 mg/L; the LC25 values were almost in an agreeable range: 691.83 to 831.76 mg/L; LC50 values were in the range: 1174.9 to 1348.9 mg/L; LC75 values were in the range: 1698.2 to 2344.2 mg/L; HPC values were in the range: 2700 to 3000 mg/L; LC100 values were in the range: 3000 to 3300 mg/L, including the results obtained for comet assay attempts, for PbCl2. Similarly, The MIC value in each method were invariably 150 mg/L; the LC25 values were almost in an agreeable range: 295.12 to 371.53 mg/L; LC50 values were in the range: 501.18 to 588.84 mg/L; LC75 values were in the range: 707.94 to 954.99 mg/L HPC values was 1350 mg/L in all assays; LC100 values was 1500 mg/L in all assays, including the results obtained for comet assay attempts, for lead acetate. It was recorded that each assay method monitored for cytotoxicity were in same range monitored for a chemical; but, separate ranges for each chemical were noted. Cytotoxicity and genotoxicity values due to each chemical were in the same range for a chemical.
Many toxicology test systems are undependable in animal systems as the exemplary mouse or rabbit or guinea pig systems are not true representative of a human body. Therefore, there are large gaps in the mirror of toxicity models with animals (Tzimas 1994); nonetheless, all popular animal models are mammals. Further, the requirement of a high number of animals in toxicology are so much for chemicals like 30,000 or more, which discourages the use of animals in assay systems (Gilbert 2010). Therefore the use of pluripotent stem cells (PSCs) and related cell lines in toxicology had been well-recognized (Rosler et al., 2004). It is consensus that, the animal system has a holistic approach in toxicity studies that is unavailable in cell cultures — a fact that supports the use of whole animal models.
However, animals tests can never be adequately standardized like human cellular systems, as the experiment should have growing cells under controlled conditions – in vitro human cell lines. In fact, the use of PSCs and their derivatives in toxicology has been well recognized (reviewed by Laustriat et al., 2010). PSCs have unique capacity of self-renewal in differentiation with advantages over somatic cells and those could be grown in vitro, as permanent cell lines. Moreover, surplus embryos from an in vitro fertilization unit could lend to the initiation of individual cell lines (Loser et al., 2010), during toxicity testing. Reprogramming murine fibroblasts by viral transfer of 4 genes, associated with pluripotency could be induced, thus PSCs could be created (Takahashi & Yamanaka, 2006). These workers could demonstrate that these specialized somatic cells could be reversed in PSCs in vitro.
The use of induced pluripotent stem cells (iPSCs) is a reliable technology for the development of different cell lines. The use of iPSC along with bio-monitoring data could enable a proper understanding of environmental metal/chemical toxicity. Thus, the iPSC technology has a tremendous potential for the development of predictive toxicology. Developmental toxicity has slowly become an important area of future assessment (Laustriat et al., 2010). Indeed, we are unaware of rising frequencies of many ailments, for example, cancer, autism, changes in pubertal timings are the areas not clearly known, with problem from environmental pollution, for example. In fact, if the toxicity pathways that induce DNA damage and affect DNA repair and other associated genetic process, predictive toxicology would bring new ideas in cancer mechanism due to chemicals of environmental concerns that could aid in the identification and classification of carcinogens (Grimsrud & Andersen, 2010). By the by, toxic effect on immune pathways could be elucidated (Guzik et al., 2001). Genotoxicity testing of 131-radioiodine a drug used for treatment of patients with thyroid diseases with cultured human lymphocytes was recently described (Hosseinimehr et al., 2013), as a work in predictive toxicology.
It has been consensus that hematopoietic /progenitor cells are more in UCB compared to bone marrow blood, as the later is involved in adults. Thus, this toxicity work using cord blood lymphocytes overrides the use of bone marrow stem cells in studies with predictive toxicology. In a study with human UCB, it was estimated that contents of nucleolar cells, granulocyte macrophage, erythroid and multipotent progenitor cells are mainly viable for at least three days under 4 °C or 21 °C (Broxmeyer et al., 1989). This study involves storage of the cells at 4 °C and further work at 22 °C. Thus, essential constituents of cord blood relating to stem cell would not be destroyed during the period of the study. Moreover, microarray technology involving environmental toxicants for understanding the underlying toxicity has been defined; to identify toxicant with specific genetic markers, such studies with heavy metals have been initiated (Bartosiewicz et al., 2001). Indeed, metals cause oxidative stress in cells, which is well studied in microarray system.
Conclusion
Lead acetate with the LC100 value around 1500 mg/L was more toxic than lead chloride with the LC100 value around 3300 mg/L to human lymphocytes. LC50 values obtained by probit analyses were accordingly different for each chemical. Cyto-toxicity and Geno-toxicity values due to each chemical were in the same range for a chemical.
Acknowledgements
R Patnaik is an INSPIRE fellow (IF 120548) of Department of Science & Technology, Govt. of India, New Delhi. Cell culture facilities were extended by IMS & Sum Hospital, Bhubaneswar.
REFERENCES
- Ahmed YF, Eldebaky HAA, Mahmoud KGM, Nawito M. Effects of lead exposure on DNA damage and apoptosis in reproductive and vital organs in female rabbits. Global Veterinaria. 2012;9:401–408. [Google Scholar]
- Anonymous. Toxicological profile for lead. Atlanta, US: US Department of Health and Human Services; 2007. Agency for toxic substances and disease registry (ATSDR) [Google Scholar]
- Bartosiewicz M, Penn S, Buckpitt A. Applications of gene arrays in environmental toxicology: fingerprints of gene regulation associated with cadmium chloride, benzo(a)pyrene, and trichloroethylene. Environ Health Persp. 2001;109:71–74. doi: 10.1289/ehp.0110971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Very low lead exposures and children’s neurodevelop-ment. Curr Opin Pediatr. 2008;20:172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Berrahal AA, Nehdi A, Hajjaji N, Gharbi N, Saloua N, EI-Fazaa S. Pharmacology, toxicology antioxidant enzymes activities and bilirubin level in adult rat treated with lead. Comptes Rendus Biol. 2007;330:581–588. doi: 10.1016/j.crvi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Bishayi B, Sengupta M. Synergism in immunotoxicological effects due to repeated combined administration of arsenic and lead in mice. Int Immunopharmacol. 2006;6:454–464. doi: 10.1016/j.intimp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. PNAS (USA) 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn TL, Ladics GS, Holsapple MP, Dietert RR. Developmental immunotoxicology assessment in the rat: age, gender and strain comparisons after exposure to Pb. Toxicol Methods. 2001;11:41–58. [Google Scholar]
- Campbell JR, Rosier RN, Novotny L, Puzas JE. The association between environmental lead exposure and bone density in children. Environ Health Perspect. 2004;112:1200–1203. doi: 10.1289/ehp.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand BD, Saya RN, Usha RM, Rajarami RG. Age related changes in aminergic system and behavior following lead exposure: protection with essential metal supplements. Neurosci Res. 2014;78:81–89. doi: 10.1016/j.neures.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Conterato GMM, Quatrin A, Somacal S, Flores EMM, Emanuelli T. Acute exposure to low lead levels and its implications on the activity and expression of cytosolic thioredoxin reductase in the kidney. Basic Clinical Pharmacol Toxicol. 2014;114:476–484. doi: 10.1111/bcpt.12183. [DOI] [PubMed] [Google Scholar]
- Eweis EA, EI-Beltagi HS, Abdel-Mobdy YE. Effect of lead acetate toxicity on experimental male albino rat. Asian Pacific J Trop Biomed. 2012;2:41–46. doi: 10.1016/S2221-1691(11)60187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsrud TK, Andersen A. Evidence of carcinogenicity in humans of water-soluble nickel salts. J Occup Med Toxicol. 2010;5(7) doi: 10.1186/1745-6673-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physio Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- Hosseinimehr SJ, Shafaghati N, Hedayati M. Genotoxicity induced by iodine-131 in human cultured lymphocytes. Interdiscip Toxicol. 2013;6:74–76. doi: 10.2478/intox-2013-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrar BM, Taib NT. Histological and histochemical alterations in the liver induced by lead chronic toxicity. Saudi J of Bio Sci. 2012;19:203–210. doi: 10.1016/j.sjbs.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi I, Nasr J, Zanganeh F. Protective effects of an alfalfa aqueous extract on lead toxicity in broiler chickens: A biochemical approach. Comp Clinical Pathol. 2013;22:1129–1136. [Google Scholar]
- Laustriat D, Gide J, Peschanski M. Human pluripotent stem cells in drug discovery and predictive toxicology. Biochem Soc Trans. 2010;38:1051–1057. doi: 10.1042/BST0381051. [DOI] [PubMed] [Google Scholar]
- Lee YJ. A case study on the effect of chelation therapy with dimercaptosuccinic acid (DMSA) for lead poisoning in an adult. Korean J Occup Environ Med. 2010;22:69–76. [Google Scholar]
- Loser P, Schirm J, Guhr A, Wobus AM, Kurtz A. Human Embryonic Stem Cell Lines and their use in international research. Stem cells. 2010;28:240–246. doi: 10.1002/stem.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa SA, Bashandy SA, Romanian J. Biophysical and biochemical changes in the blood of rats exposed to lead toxicity. Biophysics. 2008;18:123–133. [Google Scholar]
- Mujaibel LM, Kilarkaje N. Mitogen-activated protein kinase signalling and its association with oxidative stress and apoptosis in lead-exposed hepatocytes. Environ Toxicol. 2013 doi: 10.1002/tox.21928. [DOI] [PubMed] [Google Scholar]
- Park JU, Oh SW, Kim SH, Kim YH, Park RJ, Moon JD. A study on the association between blood lead levels and habitual tobacco and alcohol use in Koreans with no occupational lead exposure. Korean J Occup Environ Med. 2008;20(3):165–173. [Google Scholar]
- Rath S, Sahu MC, Dubey D, Debata NK, Padhy RN. Which value should be used as the lethal concentration 50 (LC50) with bacteria? Interdisc Sci Comput Lif Sci. 2011;3:138–143. doi: 10.1007/s12539-011-0081-x. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Richards JG, Wood CM. Ionoregulatory disruption as the acute toxic mechanism for lead in the rainbow trout (Oncorhynchus mykiss) Aquatic Toxicol. 2003;64:215–234. doi: 10.1016/s0166-445x(03)00053-5. [DOI] [PubMed] [Google Scholar]
- Rosler ES, Fisk GJ, Ares X, Irving J, Miura T, Rao MS, Carpenter MK. Long-term culture of human embryonic stem cells in feeder-free conditions. Dev Dynam. 2004;229:259–274. doi: 10.1002/dvdy.10430. [DOI] [PubMed] [Google Scholar]
- Samarghandian S, Borji A, Afshari R, Delkhosh MB, Gholami A. The effect of lead acetate on oxidative stress and antioxidant status in rat bron-choalveolar lavage fluid and lung tissue. Toxicol Mechan Meth. 2013;23:432–436. doi: 10.3109/15376516.2013.777136. [DOI] [PubMed] [Google Scholar]
- Shalan MG, Mostafa MS, Hassouna MM, Hassab SE, El-Nabi, El-Rafaie A. Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology. 2005;206:1–15. doi: 10.1016/j.tox.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Sharma V, Sharma A, Kansal L. The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem Toxicol. 2010;48:928–936. doi: 10.1016/j.fct.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Soltaninejad K, Kebriaeezadeh A, Minaiee B, Ostad SN, Hosseini R, Azizi E, et al. Biochemical and ultrastructural evidences for toxicity of lead through free radicals in rat brain. Hum Exp Toxicol. 2003;22:417–423. doi: 10.1191/0960327103ht385oa. [DOI] [PubMed] [Google Scholar]
- Sun X, Xie Y, Wu L, Zhu W, Hu J, et al. Lead acetate reduces the ability of human umbilical cord mesenchymal stem cells to support hematopoiesis in vitro. Molec Med Rep. 2012;6:827–832. doi: 10.3892/mmr.2012.1014. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–67. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tzimas G, Thiel R, Chahoud I, Nau H. The area under the concentration–time curve of all-trans-retinoic acid is the most suitable pharmacokinetic correlate to the embryotoxicity of this retinoid in the rat. Toxicol Appl Pharmacol. 1997;143:436–444. doi: 10.1006/taap.1997.8105. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency US Environmental Protection Agency (USEPA) United States Environmental Protection Agency Region 2. 2001. EPA-902-B01-001: best management practices for lead at outdoor shooting ranges EPA-902-B01-001. [Google Scholar]
- Wang X, Wang M, Dong W, Piao F, Li S. Subchronic exposure to lead acetate inhibits spermatogenesis and downregulates the expression of Ddx3y in testis of mice. Reprod Toxicol. 2013;42:242–250. doi: 10.1016/j.reprotox.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Jia CC, Lin L, Bai CL, Huang CP. Toxic effects of lead on embryonic development of zebrafish. Acta Anatomica Sinica. 2012;43:662–666. [Google Scholar]