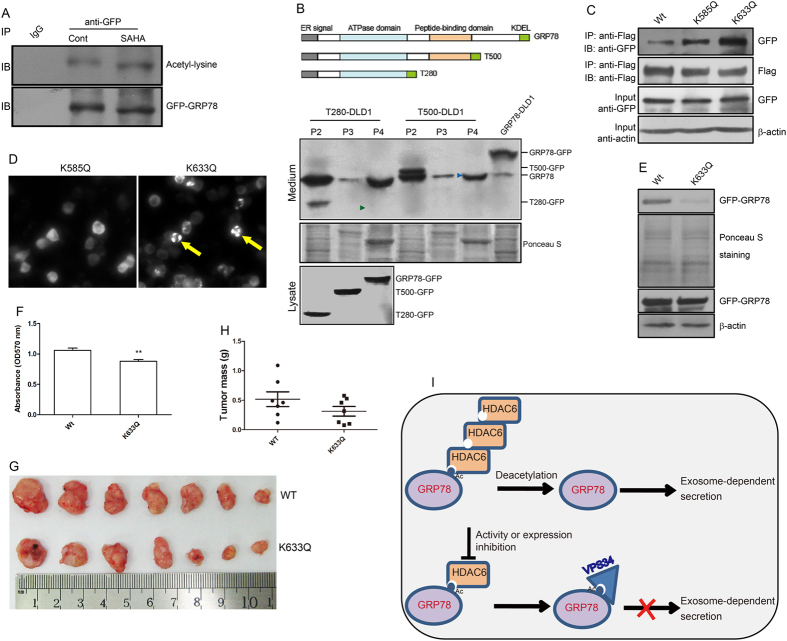
Figure 7. GRP78 acetylation affects its secretion and tumour growth.
(A) Culture supernatants from DLD1 cells stably expressing wild-type GRP78 or deletion mutants were collected and subjected to differential centrifugation, as described in Fig. 1. The P2–P4 pellets were analysed by immunoblotting for GFP. (B) Cell lysates from GRP78-GFP-expressing cells treated with or without SAHA were immunoprecipitated with an anti-GFP antibody, and an immunoblot was performed with an anti-acetyl-lysine antibody. (C) Wild-type GRP78, the K585Q mutant or the K633Q mutant was co-transfected with the VPS34-Flag plasmid into 293T cells, and lysates were immunoprecipitated with an anti-Flag antibody followed by immunoblotting with an anti-GFP antibody. Ten percent of the total cell lysate used in immunoprecipitation was used as the input. (D) The K585Q and K633Q mutants were transfected into 293T cells, and the distribution of green fluorescence was observed by fluorescence microscopy. (E) Culture supernatants from DLD1 cells expressing wild-type GRP78 and the K633Q mutant were collected and subjected to an analysis of GRP78 secretion by Western blotting. (F) An MTT assay was used to assess the growth of cells expressing wild-type GRP78 and the K633Q mutant. (G,H) Equal numbers of wild-type GRP78- and K633Q mutant-expressing cells were injected into immunocompromised mice. The dissected tumours, in pairs, and the bar graph representing the tumour weight are shown. (I) A schematic representation of the proposed model. In tumour cells highly expressing HDAC6, GRP78 is deacetylated and enters an exosome-dependent secretory pathway; in cells expressing low amounts of HDAC6 or upon inhibition by HDAC inhibitors, GRP78 cannot be deacetylated by HDAC6. Acetylated GRP78 is thus recruited to the VPS34 complex, preventing GRP78 sorting into the exosomes.