Figure 2.
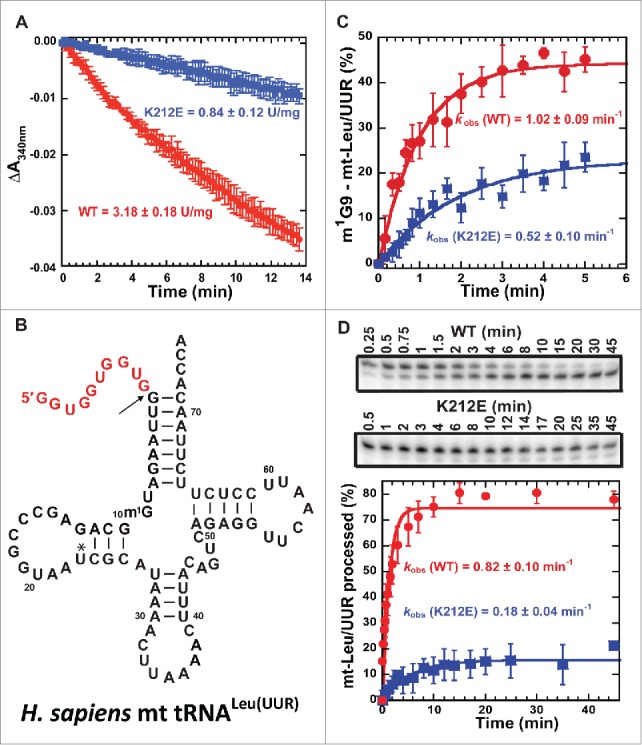
Impairment of SDR5C1-dependent activities by the p.K212E mutation. (A) The mutational effect on the dehydrogenase activity of SDR5C1, showing the rate constant of NADH oxidation to NAD+ upon reduction of acetoacetyl-CoA to 3-hydroxy-acetyl-CoA. (B) The sequence and cloverleaf structure of human mt-tRNALeu(UUR). The mature sequence is in black, whereas the 5′-precursor sequence is shown in red. (C) The mutational effect on the synthesis of m1G9-mt-tRNALeu(UUR), showing the kobs of methyl transfer of the TRMT10C-SDR5C1 sub-complex with wild-type (WT) and p.K212E mutant of SDR5C1. (D) The mutational effect on the 5′-processing activity of mitochondrial RNase P to convert the precursor of mt-tRNALeu(UUR) to the mature form, showing a time-course of the processing activity (top) and the kobs of the processing by the WT and p.K212E mutant forms of SDR5C1 in the holo-complex (bottom).
