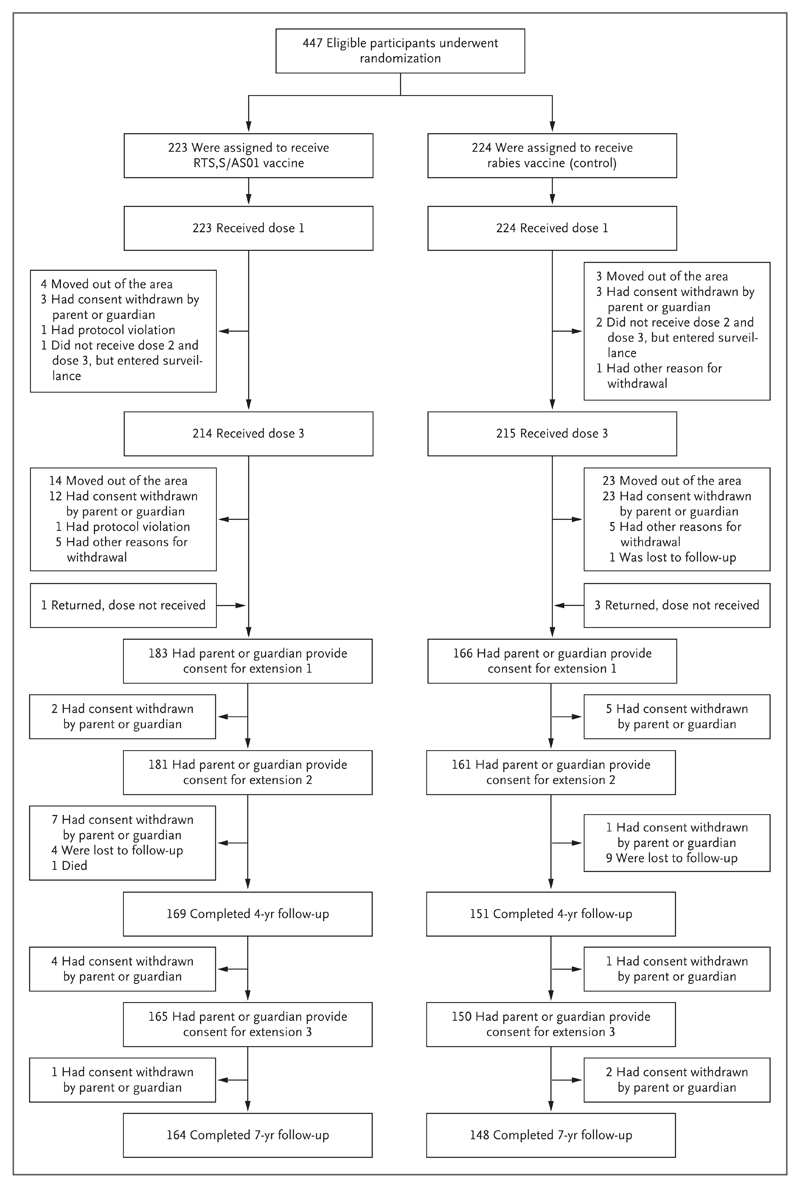
Figure 1 (facing page). Randomization and Follow-up of Trial Participants.
We conducted three extensions: from the end of 12 months of follow-up until November 2008, from then until April 2011, and finally for an additional 3 years. Other reasons for withdrawal included children missing vaccinations because of hospital admission (with contraindications to further vaccination), medical conditions not permitted by the protocol, and incomplete documentation regarding concomitant vaccinations.