Abstract
Given the expected rapid growth of the geriatric world population (=individuals aged >65 years) to 1.3 billion by 2050, age-related diseases such as osteoporosis and its sequelae, osteoporotic fractures, are on the rise. Areal bone mineral density (aBMD) by dual-energy X-ray absorptiometry (DXA) is the current gold standard to diagnose osteoporosis, to assess osteoporotic fracture risk, and to monitor treatment-induced BMD changes. However, most fragility fractures occur in patients with normal or osteopenic aBMD, indicating that factors beyond BMD impact bone strength. Recent developments in DXA technology such as TBS, VFA, and hip geometry analysis are now available to assess some of these non-BMD parameters from the DXA image. This review will discuss the use of DXA and DXA-assisted technologies and their respective advantages and disadvantages. Special attention is given to if and how each method is indicated in the geriatric population, and the latest ISCD 2015 guidelines have been incorporated.
Keywords: ISCD, FRAX, Aging, Geriatrics
Introduction
The Geriatric Patient
For millennia, mankind has struggled with the aging of the human body. The first depictions of age-related diseases such as osteoporosis date back to ancient Egypt (2800 B.C) in which the hieroglyphic for “old” was symbolized by a bent person leaning on a staff [1•]. However, it was not until 1909 when the neologism “Geriatrics,” derived from the Greek words (geras, old age) and (iatros, physician, iatrikos, relating to the physician), was introduced by Ignatz Nascher. He suggested that “as Pediatrics covered the medicine of childhood, the field of Geriatrics should be assigned a separate place in medicine, covering the medicine of old age in order to emphasize the necessity of considering senility and its disease apart from maturity” [2]. The care of geriatric patients—usually defined as any men or women over the age of 65 [3]—has since flourished with the formation of professional societies and specialized training programs that certify dedicated geriatricians. Despite these advances, the demand for high-quality geriatric care, which includes appropriate diagnostic imaging, is higher than ever before and will continue to rise. In its latest prospect of 2015, the United Nations (UN) reported a current worldwide total of 608 million women and men aged 65 years and older [4]. By 2020, the number of people aged 65 years and older is expected to surpass the number of children younger than five years for the first time and reach a staggering 1.3 billion by 2050 [4].
Osteoporosis: Background and Epidemiology
Given this expected rapid growth in the geriatric world population, diseases and conditions that primarily impact older individuals are and will be of substantial medical and socioeconomic importance [5]. One such condition is osteoporosis (from Greek: fragile or porous bone, (oston, bone), (pore)), which afflicts about 4 % of men older than 65 years and 26 % of women older than 65 years [6].
As osteoporosis is an age-related disease, nearly 75 % of osteoporotic fractures occur in individuals aged 65 years and older [7], and the lifetime risk of sustaining an osteoporotic fracture in women aged 50 years and older is 50 % [8]. Although osteoporosis is generally regarded as a disease of women, the lifetime risk of osteoporotic fracture in men is also remarkable: 27 % of men older than age 50 years will sustain an osteoporotic fracture in their remaining lifetime [9]. This is more than double the male lifetime risk of developing prostate cancer (11.3 %) [10]. As longevity continues to increase globally, the lifetime risk of osteoporotic fractures will also continue to rise [11]. With 8.9 million osteoporotic fractures estimated to occur annually worldwide, an osteoporotic fracture occurs across the globe every 3 s [12]. These estimates are concerning because osteoporotic fractures precipitate a rapid downward spiral in physical health: 20 % of all patients sustaining a hip fracture will die within 1 year after their trauma and 20 % will need permanent nursing home care [8]. Furthermore, in addition to this significant morbidity and mortality associated with osteoporotic fractures, treatment of osteoporotic fractures has significant economic ramifications. For example, by 2025, treatment costs for osteoporotic fractures in the U.S. alone are expected to exceed $25 billion [5].
Osteoporosis: Definition and Diagnosis
During the last century, several definitions for osteoporosis have been proposed, and this can be confusing for anyone new to the field (see for further review the recent article of Lorentzon et al. on the evolution of diagnosis of osteoporosis [13••]). The most widely accepted definition of osteoporosis that is still in use was issued in 2000 by a National Institutes of Health (NIH) expert panel [14]. According to this so-called “NIH consensus osteoporosis definition,” osteoporosis is a skeletal disorder that is characterized by a systemic impairment of bone strength predisposing a person to an increased risks of fragility fractures [14]. The definition further suggests that the determinants of bone strength encompass not only bone mineral density (BMD) but also bone quality. Bone mineral density is generally measured in absolute values as grams mineral Hydroxyapatite (HA), per area of bone (=areal BMD as measured by DXA, see below), or per volume of bone (volumetric BMD as measured by quantitative computed tomography (QCT), or other peripheral QCT methods such as high-resolution peripheral quantitative computed tomography (HR-pQCT)). However, BMD can also be expressed as standard deviation (SD) scores which allow one to compare the individual's results to a reference range. The umbrella term bone quality describes the set of characteristics that influence bone strength such as bone macroarchitecture (geometry: size and shape), bone microarchitecture (trabecular architecture, cortical thickness/porosity), bone turnover, accumulation of bone damage (e.g. localization, extent of micro-fractures), and mineralization [14].
Since there is currently no accurate measure of overall bone strength, BMD measurements derived from dual-energy X-ray absorptiometry (DXA) testing (see below) are currently used as the universal gold standard to diagnose osteoporosis. For this purpose, the World Health Organisation (WHO) published in 1994 the criteria that clarify under which circumstances the diagnosis of osteoporosis should be established [15]: these criteria are based on T-scores of aBMD by DXA. A T-score indicates how many standard deviations (SD) of aBMD an individual's aBMD is apart from the aBMD of a young, healthy, sex- and ethnicity-matched reference population. For example, a T-score of −3.5 in a 70-year-old patient signifies that the patient's aBMD by DXA is 3.5 standard deviations below the BMD of a healthy young reference population of the same sex and ethnicity. According to the WHO criteria, a T-score less than −1 and greater than −2.5 should be defined as osteopenia, while the diagnosis of osteoporosis should be made only if the T-score is −2.5 or lower [15]. Initially, this WHO definition was developed only for postmenopausal women. However, the International Society for Clinical Densitometry (ISCD) has modified and expanded the definition to men as well. Thus, in its latest official position of June 2015, the ISCD recommends that a diagnosis of osteoporosis should be made “in postmenopausal women and in men age 50 or older if the T-score (by DXA) of the lumbar spine, total hip or femoral neck is −2.5 or less” [16••] (see also http://www.iscd.org/official-positions/2015-iscd-official-positions-adult/). ISCD further recommends that BMD may be measured at either hip and that the lowest T-score of either site, the femoral neck or the total proximal femur, should be reported. Other hip regions of interest such as Ward's area or the greater trochanteric area should not be used for diagnosing osteoporosis [16••]. Although aBMD by DXA (as expressed by T-scores or absolute areal BMD values) can be regarded as the main parameter for diagnosing osteoporosis, the diagnosis of osteoporosis should also be made clinically as soon as one or more low-impact fractures occur, even in the absence of BMD measurements [17–19]. A low-impact or fragility fracture is defined as any fracture that results from a low-energy trauma as it occurs when falling from standing height or less or from a bone that breaks under conditions that would not cause a normal bone to break [20]. Thus, the presence of a low-energy trauma fracture is diagnostic for osteoporosis, irrespective of the patients’ BMD. Interestingly, many fragility fractures are sustained in individuals with DXA-derived aBMD in either normal or in the osteopenic range [21–23], suggesting that aBMD by DXA reflects only one facet of overall bone health and that other indicators of bone health (e.g., clinical factors and bone structural measures such as bone geometry or bone microarchitecture) should also be considered for fracture risk assessment. In this review, the use of state-ofthe-art imaging techniques for osteoporosis such as dual-energy X-ray absorptiometry (DXA) are discussed and the use of recently developed DXA-assisted technologies such as Trabecular Bone Score (TBS), Vertebral Fracture Assessment (VFA), and hip geometry analysis are reviewed. In light of the latest 2015 ISCD guidelines, special attention is given to if and how each method is indicated in the geriatric population.
Assessment of Fragility Fractures on Radiographs
Standard skeletal radiographs can be used to diagnose osteoporotic fractures and in particular vertebral fractures. However, the effective dose of a lateral radiograph of the lumbar and thoracic spine is approximately 600 μSv [24]. Another shortcoming is that radiographs are not sensitive enough to detect early osteoporotic changes before they result in fractures or to quantify these changes objectively. Estimates suggest that 20–30 % of the bone mineral must have been removed before an experienced radiologist will subjectively “recognize” osteoporosis [25••].
Dual-Energy X-ray Absorptiometry (DXA)
In order to overcome these shortcomings, dual-energy X-ray absorptiometry (DXA) was introduced in the mid-1980s [26–29]. Since then, DXA has established itself as the most widely used bone densitometry technique and as the gold standard in assessing skeletal health in clinical routine. This is mainly due to its pertinent advantages: it is a low-cost, easy-to-use, well-standardized technique, that allows in vivo quantification of aBMD of the central and peripheral skeleton with short scan times (10–30 s), high precision (max. acceptable precision error is 2–2.5 %), and with low radiation doses for the patient (effective doses vary between 1 and 20 μSv, depending on the manufacturer and the scan mode [30]). For the patient's benefit, patients can eat normally on the day of the DXA exam and can take their medication on the day of the scan as usual. However, they should refrain from taking any calcium containing tables for at least 24 h before the exam, as these tablets can overly the spine and cause false BMD elevations. In addition, patients should not have undergone within the last 10–14 days any examinations where they received radio-contrast media orally or intravenously (such as barium or iodine based compounds during a contrast CT), as these contrast agents can also affect BMD measurements.
Unlike its predecessor DPA (dual photon absorptiometry), which needed radioactive gadolinium to generate the low-energy photon beams [31], DXA itself uses an X-ray tube to generate two energy X-ray beams of different energies: one “low-energy” X-ray beam of 40 keV and one “high-energy” X-ray beam >70 keV. Using two energy beams enables subtraction of the soft tissue component [32]. Both energy beams are then transmitted through the human body, where they become attenuated differently and the remaining attenuation energies of both beams are recorded by a flexible detector arm [33•]. The areal BMD values in g/cm2 are next calculated for the scanned region of interest (ROIs): these can include the proximal femur (with its subregions of total femur region, femoral neck, intertrochanteric, and trochanteric region), the lumbar spine, and the distal radius (see Fig. 1). According to the latest ISCD 2015 official positions, DXA measurements should be performed in all patients at both sites, the spine and the hip [16••]. The forearm should only be scanned if the hip or spine cannot be measured (e.g., due to total joint replacement or degenerative changes), or in hyper-parathyroid or very obese patients (over the weight limit for the DXA table) [16••]. For evaluation of the spine region of interest, the ISCD 2015 recommends using all evaluable vertebrae and excluding only those vertebrae that are affected by local structural changes or artifacts. aBMD-based diagnostic classification should not be made using a single vertebra [16••]. For assessment of hip aBMD, aBMD may be measured at either hip. Additionally, femoral neck or total proximal femur BMD should be used whichever is lowest. Further instructions for correct patient positioning, evaluation, and reporting of DXA scans can be found under [16••, 34] or in the officially recommended ISCD study material [35, 36].
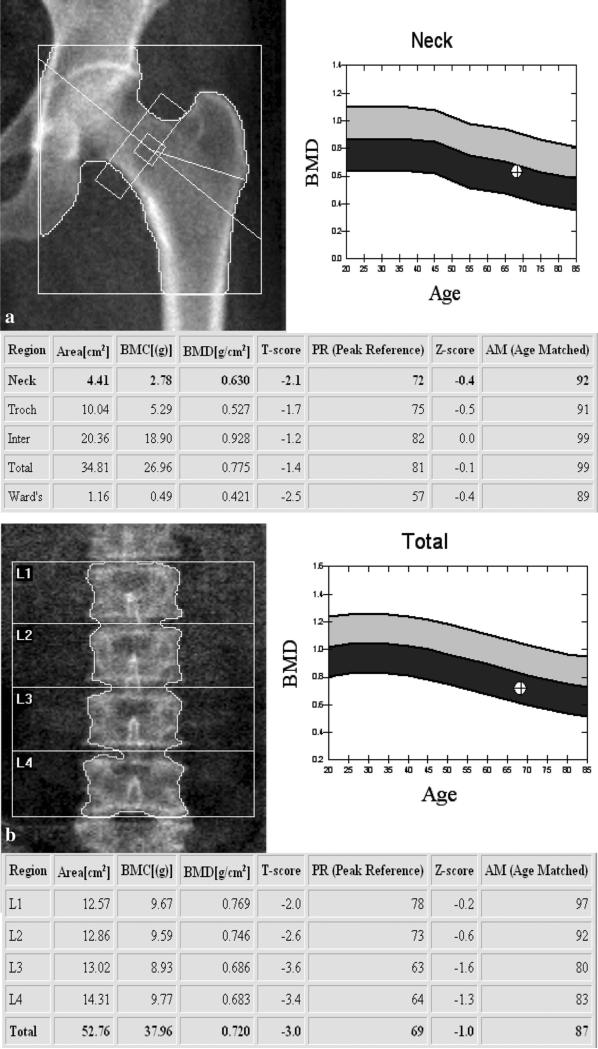
Fig. 1.
Representative DXA studies of a the proximal femur and b the lumbar spine of a 68-year-old woman. The lowest T-score of total and femoral neck regions of interest is used to grade the bone as normal, osteopenic, or osteoporotic. a In this postmenopausal woman, the T-score of the proximal femur was −2.1 which is in the osteopenic range according to the WHO osteoporosis definition. b L1–4 DXA image of the lumbar spine in the same postmenopausal women (68 years). The total T-score of the lumbar spine involving L1 to L4 is −3.0 and therefore in the osteoporotic range. Please note the discordance between the two T-scores as they fall into different, but adjacent classes (osteopenic versus osteoporotic). Priority is given in this case to the osteoporotic T-score and the patient should be diagnosed as osteoporotic
Areal BMD by DXA has been shown to strongly correlate with biomechanically assessed bone strength (about 70 % of the bone strength are explained by BMD) [14]. Even more importantly, aBMD measurements by DXA emerged as strong predictors of fracture risk, in both women and men [37, 38], and the predictive ability of spine and femoral neck aBMD by DXA remained strong even in women aged 80 years and over [39]. For example, one SD decrease in aBMD of the proximal femur increases the relative risk of sustaining a hip fracture in women aged 65–79 years by 190 %, and in women aged 80 years and over by 110 % [39]. These data suggest that aBMD measurements by DXA are useful for all geriatric patients and even in the very elderly population and that in particular this population segment may benefit most from prevention and treatment of osteoporosis. ISCD 2015 official positions reflect these findings and recommend that aBMD testing by DXA is indicated in any women aged 65 years and older and in any men aged 70 years and older. For any men <70 years of age, BMD testing is also indicated if he experiences an additional risk factor for low bone mass. Such a risk factor includes low body weight (BMI of <20 kg/m), a prior fragility fracture, high-risk medication use (e.g., the ever use of corticosteroids) or any disease or condition associated with bone loss [16••, 40]. As treatment of osteoporosis can markedly reduce fracture risk [41] even in very elderly populations [39, 42], follow-up DXA measurements every 1–2 years are frequently carried out in clinical routine to monitor treatment response. However, controversy surrounds the indications and recommended frequency of repeat DXA testing [43]. Unfortunately, the current ISCD official position of 2015 lacks to comment on when and at which frequency follow-up DXA scans are indicated, and leaves it up to the reporting physician of the initial DXA report to comment on the necessity and timing of potential follow-up scans. ISCD does, however, point out that any follow-up DXA report should include a statement about the least significant change (LSC)—a measure of reproducibility that is specific to the DXA facility where BMD measurements are being carried out. If the measured change in aBMD between baseline and follow-up DXA scan is larger than the provided LSC, the BMD change should be considered as genuine, and treatment alterations may be enacted [44, 45].
DXA does have some notable limitations. First and foremost, DXA is only a two-dimensional (2D) technique that quantifies only areal BMD (in grams per square centimeter). DXA does not provide volumetric (3D) BMD measurements (in milligrams per cubic centimeter) as they can be derived from any quantitative computed tomography techniques such as QCT, pQCT, or HR-pQCT. In addition, DXA does not offer information about the three-dimensional bone microstructure/architecture (e.g., cortical porosity) nor does it differentiate cortical and trabecular BMD. Areal BMD by DXA is also dependent on bone and body size, leading to erroneous overestimation of fracture risk in small patients [46]. Obesity, on the other hand, goes along with larger precision errors and reduced reproducibility [47, 48]. This needs to be considered when interpreting DXA results of obese patients, particularly of patients with larger changes in body composition such as bariatric patients in which hip aBMD by DXA seems specifically prone to weight loss-induced artifacts [49]. Proper patient positioning and image analysis are also critical: spine and hip DXA aBMD measurements are susceptible to degenerative changes which are very common in the elderly (e.g., lumbar spine osteophytes or aortic calcifications) and will falsely elevate BMD and underestimate the true fracture risk in these patients [50]. Moreover, it is crucial to check DXA images for artifacts (e.g. surgical clips, radio-opaque tablets, residuals of radiopaque contrast dye) as they can impact BMD results [51].
Fracture Risk Assessment Tool (WHO-FRAX)
Because of these limitations and in order to improve fracture prediction, the World Health Organization launched in 2008 the WHO Fracture Risk Assessment tool (FRAX), a web-based tool available online under (www.shef.ac.uk/FRAX/) or downloadable as mobile and desktop application under (www.iofbonehealth.org/osteoporosis-musculoskeletal-disorders/osteoporosis/diagnosis/frax-information-and-resources). This web tool combines clinical information on well-validated osteoporotic risk factors with DXA aBMD of the femoral neck in order to predict the 10-year probability of hip fracture and major osteoporotic fractures (wrist, humerus, hip, and clinical spine fracture) for both women and men [52, 53]. Eleven clinical risk factors are integrated in the FRAX algorithm including age, sex, weight, height, history of previous fracture and of rheumatoid arthritis, history of parental hip fracture, smoking, alcohol use, glucocorticoids, and disorders associated with secondary osteoporosis. Since fracture probability varies notably between different regions of the world (e.g., countries like Iceland and Denmark have a very high fracture risk and countries like Turkey and Chile have a low fracture risk [54]), separate FRAX models are available for different countries and different geographic regions (Europe, Asia, the Middle East, Africa, as well as Northern and Latin America). It is important that the user first calibrates the FRAX model to the patient's home country, or, if this is not possible, to a surrogate country of the same fracture risk epidemiology before entering the clinical information [53]. The latest guidelines issued by the National Osteoporosis Foundation recommend starting osteoporotic treatment in those postmenopausal women and men age 50 and older who demonstrate low bone mass by DXA (T-score between −1.0 and −2.5, osteopenia) at the femoral neck, total hip, or lumbar spine and show a 10-year FRAX hip fracture probability >3 % or a 10-year FRAX major osteoporosis-related fracture probability of >20 % (these probabilities are based on the U.S.-adapted WHO absolute fracture risk model) [55]. Although the worldwide usage of FRAX is steadily increasing with more than 2 million calculations per index year [56], a recent meta-analysis reported only a fair performance in predicting the 10-year probability of hip fractures for FRAX without aBMD (women AUC of 0.74, men AUC of 0.71) and for FRAX with aBMD in both genders (in women AUC of 0.79, men 0.71) [57]. These findings demonstrate that clinical fracture risk assessment and DXA-derived aBMD as combined in FRAX cannot capture the full extent of hip fragility fractures. Other readily available and non-invasively measureable determinants of bone strength such as bone geometry and bone microarchitecture need to be taken into account to more reliably predict osteoporotic fracture risk.
Adjuncts to Standard DXA Testing: TBS, VFA, and Hip Geometry Analysis
In an attempt to achieve a more accurate identification of individuals at higher fracture risk using readily available clinical techniques such as DXA, several novel tools all based upon DXA images have been developed over the last few years. These adjuncts to standard DXA testing include the (vertebral) trabecular bone score (TBS), the vertebral fracture risk assessment (VFA), as well as DXA-derived hip geometry parameters.
Trabecular Bone Score (TBS)
First described in 2008 by Pothuaud et al. [58], Trabecular Bone Score is a gray-level textural index that can be derived from standard lumbar spine two-dimensional DXA images using dedicated software (TBS iNsight, Medimaps, Plan-les-Quates, Switzerland) [59•]. It relies on the principle that a lumbar DXA image is a 2D projection of the scanned spine and its vertebra and that it can, therefore, be decomposed in thousands of two-dimensional pixels (picture elements), each of which has an assigned gray value. As trabeculae are rarified in an osteoporotic vertebra, the 2D-projection of such a vertebra will generate an image with a low number of pixel-to-pixel gray variations, but of high amplitude (see for further detail Silva et al. [59•]). In healthy bone instead, the trabeculae form a dense mesh that produces, when projected onto a 2D plane, an image with a large number of pixel-to-pixel variations of small amplitude. By assessing the gray value and the degree of spatial dependency for each pixel and its neighboring pixels, the TBS software calculates a variogram for each vertebra as the sum of the squared gray-level differences between pixels at a specific distance. TBS (unitless) is then computed as the slope of the log–log transform of this variogram with the slope characterizing the rate of gray-level amplitude variations [59•]. A dense trabecular structure results in a 2D image with a high number of pixel gray value variations and therefore a steep variogram slope and a high TBS value. Porous, osteoporotic trabecular bone produces a low variogram slope and consequently, a low TBS value [59•, 60]. TBS software has been FDA approved (http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K121716) and can be installed on the latest generation of DXA scanners (Prodigy and iDXA of GE Lunar, Madison, WI, or Discovery, Delphi, QDR4500, and Horizon models from Hologic, Bedford, MA; no compatibility with Hologic Explorer). TBS values are usually reported for each vertebra separately and for the total spine (L1–L4). Analogous to DXA-derived lumbar BMD measurements, abnormal or fractured vertebrae can be excluded from the TBS analysis. Retrospective analysis of DXA images is also possible and not time consuming (~5–10 min per scan), once the DXA scanner, from which the DXA images have been obtained, is calibrated with a phantom.
Recent clinical studies have repeatedly demonstrated that low TBS is associated with a raise in both prevalent and incident fragility fractures in postmenopausal women [61–68] and older men [69–71]. Furthermore, this predictive ability of TBS is independent of clinical risk factors, FRAX, and DXA aBMD [72]. It is, however, noteworthy that after adjustment for confounders, TBS and lumbar spine aBMD by DXA predicted fractures equally well [68]. In light of these findings and to provide guidance for clinicians, the ISCD has recently for the first time included recommendations on the use of TBS into its 2015 official positions [16••]. Therein, the ISCD acknowledges TBS as being associated with vertebral, hip and major osteoporotic fracture risk in postmenopausal women and with hip and major osteoporotic fracture in men over the age of 50 years. In line with a recent meta-analysis [73], the ISCD further recommends that TBS can be used in association with FRAX and aBMD to adjust the FRAX probability of fracture in postmenopausal women and older men. Such a possibility now exists, as TBS has recently been implemented onto the WHO-FRAX website. However, the ISCD clearly states that TBS should not be used as single measurement to determine therapeutic recommendations [16••]. The ISCD also points out that TBS is not useful for monitoring bisphosphonate therapy in postmenopausal osteoporotic women [16••, 74].
This caution towards TBS is derived from several pertinent disadvantages. First, TBS cutoff values that would allow identification of those subjects at greatest risk of fracture have not been established. Secondly, although TBS claims to be an indirect measure of trabecular bone structure, it is currently still not clear to which extent TBS reflects the status of bone microarchitecture in a patient [74, 75]. A handful of small ex vivo studies have demonstrated correlations between TBS and trabecular microarchitectural parameters such as trabecular number, trabecular spacing, and connectivity density [76–78]. However, human in vivo validation studies are scarce and are either focused on a comparison of TBS with central bone microarchitectural parameters of limited resolution (as obtained by QCT measures) or on comparing TBS with bone microstructure parameters of high resolution, but acquired from peripheral skeletal sites such as with HR-pQCT [79–81]. In addition, TBS showed in these studies only weak correlations to vBMD and trabecular indices at the radius and tibia [82], some of them turning even insignificant after multivariable adjustments [81]. Larger high-resolution cadaveric studies are needed to investigate the level of correlation between TBS and local vertebral microarchitecture (see also [74, 75]). In addition, TBS reproducibility studies found not only a reduced short-term intra-scan precision of TBS in comparison to BMD [83] but also revealed notable inter-scanner differences in TBS values (particularly between standard GE DXA scanners and higher resolution models such as iDXA), despite an excellent inter-scanner agreement for BMD [84]. Given these current limitations, TBS values acquired at different densitometers should not be directly compared to each other and serial TBS scans should only be obtained at the same DXA instrument [84]. Further work on quality control (development of specific TSB phantom, scanner cross-calibration) and additional clinical studies are needed to determine whether TBS will be appropriate for clinical use.
Vertebral Fracture Assessment (VFA)
Vertebral fractures are the most common osteoporotic fractures, especially in geriatric patients. Population-based studies show that the prevalence of vertebral fragility fractures in white women aged 65 years and older is about 23.5 % and rises up to 50 % for women aged ≥80 years [85, 86]. Prevalence of vertebral fracture in men is also high, ranging from about 20 % in men aged 65 years and older to over 39 % in men aged ≥80 years [85, 86]. Since a first vertebral fracture not only increases an individual's risk for subsequent vertebral fracture by fivefold [87, 88] but also increases the risk to sustain a future non-vertebral osteoporotic fracture [89], and since osteoporotic therapies can reduce the incidence of future fractures, it is crucial that vertebral fractures are detected and diagnosed correctly and as early as possible. However, spine imaging by plain radiographs is not routinely carried out in patients at risk, due to costs, accessibility, or concerns of radiation (about 600 μSv per lateral spine radiograph). In addition, only about 30 % of all vertebral fractures are symptomatic and come to clinical attention [90]. The major part of osteoporotic vertebral fractures is clinically silent and is randomly detected during imaging studies performed for other clinical reason such as lateral chest X-ray, CT, or MRI or not detected at all [91, 92]. To minimize underreporting, the International Osteoporosis Foundation (IOF) has launched the Vertebral Fracture Initiative. This free teaching program is accessible online to all interested radiologists, clinicians, and other healthcare professionals (www.iofbonehealth.org/what-wedo/training-and-education/educational-slide-kits/vertebral-fracture-teaching-program). It is dedicated to training clinicians in the proper recognition of osteoporotic vertebral fractures using different imaging techniques including conventional radiography, DXA-based Vertebral Fracture Assessment (VFA), or other spinal imaging techniques such as MDCT and MRI. Although this present review will mainly focus on VFA (see recent reviews by Link et al. [93] and Griffith et al. [94••] for further reading on the radiologic assessment of vertebral osteoporotic fractures), it is crucial to understand that the osteoporotic vertebral fracture assessment in any of the aforementioned modalities is performed using the same standardized, universally accepted, visual semi-quantitative grading system by Genant et al. [95••]. To diagnose a vertebral osteoporotic fracture with this grading system, the physician evaluates visually the shape and height of each vertebra in the sagittal plane (either lateral radio-graph/DXA-VFA image, or sagittal MDCT reformations, or sagittal MR sequence). A fracture should be reported if there is at least a ≥ 20 % visually estimated loss in vertebral body height relative to a normal looking adjacent vertebra. The reduction in vertebral body height can occur either at the anterior edge of the vertebra (leading to a wedge-shaped fracture), or in the middle of the vertebra (creating a biconcave shaped or bow tie fracture), or can involve the posterior edge of the vertebra (resulting in a fracture with crush/compression deformity). The Genant score differentiates between four fracture severities: no fracture, mild, moderate, and severe fracture. It is crucial to mention the severity of a fracture in the radiology report as vertebral fracture severity has been shown to strongly predict disease progression and mirrors the deterioration of bone microarchitecture [96, 97]. While grade 0 is equivalent to “no fracture”, a mild (or grade 1) osteoporotic vertebral fracture is defined as an approximate 20–25 % reduction in anterior, middle or posterior height or a 10–20 % reduction of vertebral area. A moderate or grade 2 fracture requires an approximate reduction of 25–40 % in vertebral height or a 20–40 % reduction in vertebral area. Severe fractures should be diagnosed if the vertebra shows more than 40 % reduction in any vertebral height or vertebral area [95••].
Recent advances in DXA technology have made it possible to evaluate the presence and severity of vertebral fractures not only on conventional radiographs, MDCT, and MRI but also on spine images acquired via DXA [98]. This densitometric lateral spine imaging technique is called vertebral fracture assessment (VFA, Fig. 2). Depending on the manufacturer, different acronyms for the same technique such as lateral vertebral assessment (LVA), anterior vertebral assessment (APVA), dual vertebral assessment (DVA), instant vertebral assessment (IVA), or radiologic vertebral assessment (RVA), circulate in the literature. However, the usage of these terms is officially discouraged by ISCD [98]. VFA uses standard DXA scanners to obtain lateral and frontal images of the entire spine in near-radiographic quality in a short amount of time (between 1 and 10 min, depending on scanner model) [99]. The vertebrae inferior to T7 (T7–L5) are then routinely assessed for fractures using the Genant grading system and via automated vertebral morphometric analysis (MXA). Like plain spine radiography, VFA can detect moderate to severe vertebral fractures with high accuracy [100], while detection of mild fracture with VFA seems less reliable relative to plain radiography [101]. Several studies have demonstrated the added diagnostic and therapeutic value of combined VFA and DXA aBMD testing [102, 103]. Olenginski et al. reported that VFA detected in 34 % of patients aged 65 years and older vertebral fractures [102]. Further evidence by Greenspan et al. demonstrated that 11–18 % of all patients would have been misclassified as “normal” by the use of aBMD alone, while the additional application of VFA resulted in the clinical diagnosis of osteoporosis and influenced patient management [103]. This and further evidence [104] suggest that VFA is a useful tool to decrease misclassification and prevent potential mismanagement of osteoporosis. This is particularly noteworthy, as VFA has certain other pertinent advantages that make it promising for clinical use, namely VFA can be conveniently performed at the time of bone densitometry testing by DXA at low cost [105]. Moreover, VFA uses only a fraction of the radiation dose incurred by plain radiography (2–50 μSv for VFA versus 600 μSv in conventional spinal radiographs [24]). Current ISCD official positions (2013 and 2015) therefore recommend lateral spine imaging with either standard radiography or with densitometric VFA in all women aged ≥70 years and all men aged ≥80 years who display a DXA T-score of less than −1.0 [16••, 106•]. Moreover, VFA or lateral spine radiography is indicated in all women and men of any age with a T-score less than −1.0 in whom one or more of the following risk factors are present: a historical height loss exceeding 4 cm (>1.5 in.) [107], a prior vertebral fracture (self-reported, undocumented) [108], and a glucocorticoid therapy equivalent to ≥5 mg of prednisone or equivalent per day for ≥3 months [109].
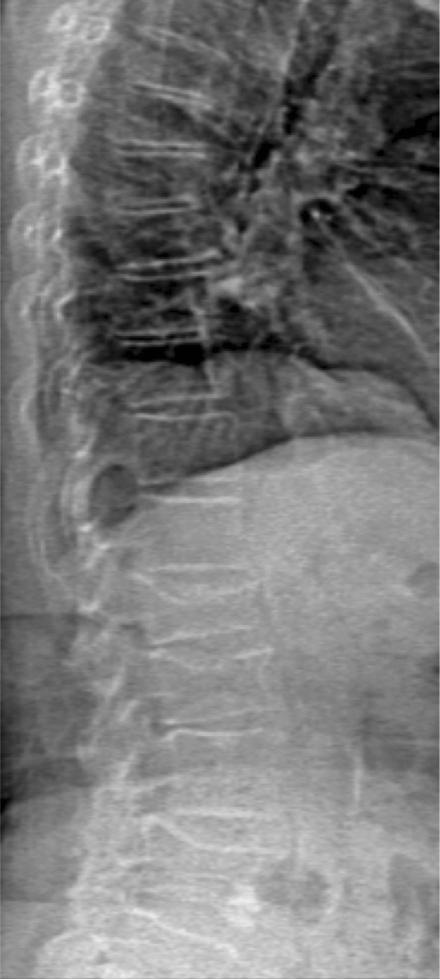
Fig. 2.
Representative vertebral fracture assessment (VFA) image of the spine obtained with DXA showing multilevel osteoporotic fractures at the lumbar spine
Hip Geometry Analysis
Several bone geometry parameters can be derived from the DXA image of the proximal femur. These include the measurement of the hip axis length (HAL, which is defined as the length of a line drawn to connect the inner pelvic brim to the lateral margin of the femoral shaft below the greater tro-chanter [110]) and the determination of the neck-shaft angle (NSA, defined as the angle between femoral neck axis and femoral midshaft axis [111]). Depending on the scanner, additional parameters such as cross-sectional area (CSA), cross-sectional moment of inertia (CSMI), buckling ratio (BR), or section modulus (SM) can be routinely obtained from DXA scans using commercially available software packages such as Hip Structural Analysis (HSA, from Hologic) [112] or Advanced Hip Assessment (AHA, from General Electrics). While HAL has been shown in multiple populations to predict the risk of hip fracture in post-menopausal women independent of BMD [110, 113, 114] and FRAX [115], data on the predictive value of HAL in men is scarce. In line with these findings, the latest ISCD guidelines of 2015 acknowledge that HAL is only a good predictor of hip fracture risk in postmenopausal women, but not in men [116]. However, a recently published study by Leslie et al. in the Manitoba cohort provides the first evidence that HAL is also predictive of incident hip fracture in both men and women, independent of BMD or FRAX [117]. Like HLA, NSA, and other hip geometry parameters including buckling ratio and section modulus have been found to predict hip fracture in postmenopausal Caucasian women [114, 118–121]. However, to date, it is not yet clear if this risk is independent of BMD. Due to this reason and other reasons [116], the latest guidelines issued by ISCD in 2015 do not support the use of NSA or any other DXA-derived hip geometry parameters (CSA, OD, DM, BR, CSMI) in clinical practice to neither assess fracture risk nor start or monitor osteoporotic treatment [116].
Conclusion
Geriatric individuals (age >65 years) currently make up 608 million of the world population and this demographic is estimated to grow to 1.3 billion by 2050. Given this expected rapid growth in the geriatric world population, the burden of age-related diseases such as osteoporosis, which affects one in two women and one in five men aged >50 -years, will be substantial. Correct and early diagnosis of osteoporosis using state-of-the-art imaging is and will be essential to start patients on anti-osteoporotic therapy and to prevent future osteoporotic fragility fractures and associated morbidity and mortality. Areal Bone mineral density (aBMD) measurement by dual-energy X-ray absorptiometry (DXA) is most widely accessible and will remain for the foreseeable future the imaging procedure of choice to diagnose osteoporosis, assess osteoporotic fracture risk, and monitor BMD changes resulting from anti-osteoporotic treatment. Due to recent advances in DXA technology, additional measurements can be extracted from DXA images, including trabecular bone score (TBS), vertebral fracture risk assessment (VFA), and hip geometry parameters such as hip length axis (HLA) or neck-shaft angle (NSA). However, the role of these DXA-assisted technologies in clinical diagnosis has still to be determined.
Acknowledgments
The authors are supported by NIH grants U01-AR059507 and P50-AR060752.
Footnotes
This article is part of the Topical Collection on Geriatrics.
Compliance with Ethics Guidelines
Conflict of Interest Ursula Heilmeier, Jiwon Youm, and Soheyla Torabi each declare no potential conflicts of interest. Thomas M. Link is the Editor-in-Chief of Current Radiology Reports.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1•.Ritch A. History of geriatric medicine: from hippocrates to marjory Warren. J R College Phys Edinb. 2012;42(4):368–74. doi: 10.4997/JRCPE.2012.417. doi:10.4997/jrcpe.2012.417. [A review article on the history of geriatrics from ancient times to Marjory Warren will illustrative examples.] [DOI] [PubMed] [Google Scholar]
- 2.Nascher JL. Geriatrics. NY Med J. 1909;90:358–9. [Google Scholar]
- 3.Besdine RW. Introduction to geriatrics. Whitehouse Station: Merck Manuals Professional Edition. Geriatrics. 2013 [Google Scholar]
- 4.United Nations . World population prospects: the 2015 revision. DVD Edition; New York: 2015. [Google Scholar]
- 5.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. doi:10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.Praemer A, Furner S, Rice DP. Musculoskeletal conditions in the United States. American Academy of Orthopaedic Surgeons; Rosemont: 1999. [Google Scholar]
- 7.Melton LJ, 3rd, Crowson CS, O’Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9(1):29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 8.Office of the Surgeon General (US) In: Bone health and osteoporosis: a report of the surgeon general. Services DoHaH, editor. Office of the Surgeon General (US); Rockville: 2004. [PubMed] [Google Scholar]
- 9.Cooley H, Jones G. A population-based study of fracture incidence in southern Tasmania: lifetime fracture risk and evidence for geographic variations within the same country. Osteoporos Int. 2001;12(2):124–30. doi: 10.1007/s001980170144. doi:10.1007/s001980170144. [DOI] [PubMed] [Google Scholar]
- 10.Merrill RM, Weed DL, Feuer EJ. The lifetime risk of developing prostate cancer in white and black men. Cancer Epidemiol Biomark Prev. 1997;6(10):763–8. [PubMed] [Google Scholar]
- 11.WHO. World health statistics. WHO; New York: 2010. [Google Scholar]
- 12.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33. doi: 10.1007/s00198-006-0172-4. doi:10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 13••.Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. J Intern Med. 2015;277(6):650–61. doi: 10.1111/joim.12369. doi:10.1111/joim. 12369. [Very nice review article that elucidates the different approaches how to define osteoporosis.] [DOI] [PubMed] [Google Scholar]
- 14.NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Technical report: assessment of fracture risk and its application to screening for postmenopausal osteoporosis: a report of a WHO study group. Geneva: World Health Organization. 1994 [PubMed] [Google Scholar]
- 16••.Shepherd JA, Schousboe JT, Broy SB, Engelke K, Leslie WD. Executive summary of the 2015 ISCD position development conference on advanced measures from DXA and QCT: fracture prediction beyond BMD. J Clin Densitom. 2015;18(3):274–86. doi: 10.1016/j.jocd.2015.06.013. doi:10.1016/j.jocd.2015.06.013. [Very important article. Summarizes the latest official positions of the International Society of Clincial Densitometry (ISCD) 2015 on the use and indications of different bone densitometry methods, such as DXA, TBS and VFA. These positions were released in June 2015 and can also be found on the homepage of the ISCD under http://www.iscd. org/official-positions/2015-iscd-official-positions-adult/.] [DOI] [PubMed] [Google Scholar]
- 17.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359(9321):1929–36. doi: 10.1016/S0140-6736(02)08761-5. doi:10.1016/s0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 18.Link TM. Osteoporosis imaging: state of the art and advanced imaging. Radiology. 2012;263(1):3–17. doi: 10.1148/radiol.12110462. doi:10.1148/radiol.12110462,10.1148/radiol.2633201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Osteoporosis Foundation . Clinician's guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation; Washington, DC: 2013. [Google Scholar]
- 20.Krueger D, Caudill J, Colquhoun A, Jankowski L. CBDT study guide. International Society for Clinical Densitometry; Middletown: 2012. [Google Scholar]
- 21.Kanterewicz E, Puigoriol E, Garcia-Barrionuevo J, del Rio L, Casellas M, Peris P. Prevalence of vertebral fractures and minor vertebral deformities evaluated by DXA-assisted vertebral fracture assessment (VFA) in a population-based study of postmenopausal women: the FRODOS study. Osteoporos Int. 2014;25(5):1455–64. doi: 10.1007/s00198-014-2628-2. doi:10.1007/s00198-014-2628-2. [DOI] [PubMed] [Google Scholar]
- 22.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286(22):2815–22. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 23.Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. 2005;20(10):1813–9. doi: 10.1359/JBMR.050609. doi:10.1359/jbmr.050609. [DOI] [PubMed] [Google Scholar]
- 24.Damilakis J, Adams JE, Guglielmi G, Link TM. Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol. 2010;20(11):2707–14. doi: 10.1007/s00330-010-1845-0. doi:10.1007/s00330-010-1845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Licata A, Williams SE. A case-based manual for understanding and interpreting bone densitometry. Springer; New York: 2014. A DXA primer for the practicing clinician. [Excellent case-based book dedicated to teach the essentials and pitfalls of interpreting and reporting DXA-exams. Very helpful training resource for residents and any other health care professionals.] [Google Scholar]
- 26.Cullum ID, Ell PJ, Ryder JP. X-ray dual-photon absorptiometry: a new method for the measurement of bone density. Br J Radiol. 1989;62(739):587–92. doi: 10.1259/0007-1285-62-739-587. doi:10.1259/0007-1285-62-739-587. [DOI] [PubMed] [Google Scholar]
- 27.Mazess R, Collick B, Trempe J, Barden H, Hanson J. Performance evaluation of a dual-energy x-ray bone densitometer. Calcif Tissue Int. 1989;44(3):228–32. doi: 10.1007/BF02556569. [DOI] [PubMed] [Google Scholar]
- 28.Wahner HW, Dunn WL, Brown ML, Morin RL, Riggs BL. Comparison of dual-energy X-ray absorptiometry and dual photon absorptiometry for bone mineral measurements of the lumbar spine. Mayo Clin Proc. 1988;63(11):1075–84. doi: 10.1016/s0025-6196(12)65502-5. [DOI] [PubMed] [Google Scholar]
- 29.Rutt B, Stebler B, Cann C. High speed, high precision dual photon absorptiometry.. 7th annual meeting of the American Society for Bone and Mineral Research; Washington, DC. 1985. [Google Scholar]
- 30.Blake GM, Naeem M, Boutros M. Comparison of effective dose to children and adults from dual X-ray absorptiometry examinations. Bone. 2006;38(6):935–42. doi: 10.1016/j.bone.2005.11.007. doi:10.1016/j.bone.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Kelly TL, Slovik DM, Schoenfeld DA, Neer RM. Quantitative digital radiography versus dual photon absorptiometry of the lumbar spine. J Clin Endocrinol Metab. 1988;67(4):839–44. doi: 10.1210/jcem-67-4-839. [DOI] [PubMed] [Google Scholar]
- 32.Blake GM, Fogelman I. Dual energy X-ray absorptiometry and its clinical applications. Semin Musculoskelet Radiol. 2002;6(3):207–18. doi: 10.1055/s-2002-36718. doi:10.1055/s-2002-36718. [DOI] [PubMed] [Google Scholar]
- 33•.Blake GM, Fogelman I. Technical principles of dual energy x-ray absorptiometry. Semin Nucl Med. 1997;27(3):210–28. doi: 10.1016/s0001-2998(97)80025-6. [Very good technical article providing a detailed description of the underlying principles of DXA. Explains also other methods such as SPA and DPA, the predecessors of DXA. In addition see also interactive online tool for DXA lectures with animations and simplified explanations under: Pintauro Stephen, Department of Nutrition and Food Sciences, University of Vermont, DEXA, Dual Energy X Ray Absorptiometry, Introduction, available under http://nutrition.uvm.edu/bodycomp/dexa/.] [DOI] [PubMed] [Google Scholar]
- 34.Jacobson JA, Jamadar DA, Hayes CW. Dual X-ray absorptiometry: recognizing image artifacts and pathology. AJR Am J Roentgenol. 2000;174(6):1699–705. doi: 10.2214/ajr.174.6.1741699. doi:10.2214/ajr.174.6.1741699. [DOI] [PubMed] [Google Scholar]
- 35.Bonnick SL. Densitometry in clinical practice: application and interpretation. 3rd ed. Humana Press; New York: 2010. [Google Scholar]
- 36.International Society for Clinical Densitometry ISCD Clinician Study Guide 2009; Version 9.1. 2009 [Google Scholar]
- 37.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–94. doi: 10.1359/JBMR.050304. doi:10.1359/jbmr.050304. [DOI] [PubMed] [Google Scholar]
- 38.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nevitt MC, Johnell O, Black DM, Ensrud K, Genant HK, Cummings SR. Bone mineral density predicts non-spine fractures in very elderly women. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4(6):325–31. doi: 10.1007/BF01622192. [DOI] [PubMed] [Google Scholar]
- 40.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, et al. Assessment of fracture risk. Osteoporos Int. 2005;16(6):581–9. doi: 10.1007/s00198-004-1780-5. doi:10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 41.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–87. doi: 10.1016/S0140-6736(10)62349-5. doi:10.1016/s0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. New Engl J Med. 1992;327(23):1637–42. doi: 10.1056/NEJM199212033272305. doi:10.1056/nejm199212033272305. [DOI] [PubMed] [Google Scholar]
- 43.Laster AJ. Dual-energy X-ray absorptiometry: overused, neglected, or just misunderstood? N C Med J. 2014;75(2):132–6. doi: 10.18043/ncm.75.2.132. [DOI] [PubMed] [Google Scholar]
- 44.Baim S, Wilson CR, Lewiecki EM, Luckey MM, Downs RW, Jr, Lentle BC. Precision assessment and radiation safety for dual-energy X-ray absorptiometry: position paper of the International Society for Clinical Densitometry. J Clin Densitom. 2005;8(4):371–8. doi: 10.1385/jcd:8:4:371. [DOI] [PubMed] [Google Scholar]
- 45.Gluer CC. Monitoring skeletal changes by radiological techniques. J Bone Miner Res. 1999;14(11):1952–62. doi: 10.1359/jbmr.1999.14.11.1952. doi:10.1359/jbmr.1999.14.11.1952. [DOI] [PubMed] [Google Scholar]
- 46.Kroger H, Vainio P, Nieminen J, Kotaniemi A. Comparison of different models for interpreting bone mineral density measurements using DXA and MRI technology. Bone. 1995;17(2):157–9. doi: 10.1016/s8756-3282(95)00162-x. [DOI] [PubMed] [Google Scholar]
- 47.Knapp KM, Welsman JR, Hopkins SJ, Fogelman I, Blake GM. Obesity increases precision errors in dual-energy X-ray absorptiometry measurements. J Clin Densitom. 2012;15(3):315–9. doi: 10.1016/j.jocd.2012.01.002. doi:10.1016/j.jocd.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27(1):119–24. doi: 10.1002/jbmr.506. doi:10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu EW, Bouxsein ML, Roy AE, Baldwin C, Cange A, Neer RM, et al. Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J Bone Miner Res. 2014;29(3):542–50. doi: 10.1002/jbmr.2063. doi:10.1002/jbmr.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenne M, McGuigan F, Besjakov J, Gerdhem P, Akesson K. Degenerative changes at the lumbar spine–implications for bone mineral density measurement in elderly women. Osteoporos Int. 2013;24(4):1419–28. doi: 10.1007/s00198-012-2048-0. doi:10.1007/s00198-012-2048-0. [DOI] [PubMed] [Google Scholar]
- 51.Watts NB. Fundamentals and pitfalls of bone densitometry using dual-energy X-ray absorptiometry (DXA). Osteoporos Int. 2004;15(11):847–54. doi: 10.1007/s00198-004-1681-7. doi:10.1007/s00198-004-1681-7. [DOI] [PubMed] [Google Scholar]
- 52.Kanis JA, on behalf of the World Health Organisation Scientific Group . Assessment of osteoporosis at the primary health care level: WHO Collaborating Centre for Metabolic Bone Diseases. University of Sheffield; Sheffield: 2007. [Google Scholar]
- 53.Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–43. doi: 10.1016/j.bone.2009.01.373. doi:10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
- 54.Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A, Ogelsby AK. International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res. 2002;17(7):1237–44. doi: 10.1359/jbmr.2002.17.7.1237. doi:10.1359/jbmr.2002.17.7.1237. [DOI] [PubMed] [Google Scholar]
- 55.National Osteoporosis Foundation . Clinician's Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation; Washington, DC: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanis JA, Johansson H, Oden A, Cooper C, McCloskey EV. Worldwide uptake of FRAX. Arch osteoporos. 2014;9:166. doi: 10.1007/s11657-013-0166-8. doi:10.1007/s11657-013-0166-8. [DOI] [PubMed] [Google Scholar]
- 57.Marques A, Ferreira RJ, Santos E, Loza E, Carmona L, da Silva JA. The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(11):1958–67. doi: 10.1136/annrheumdis-2015-207907. doi:10.1136/annrheumdis-2015-207907. [DOI] [PubMed] [Google Scholar]
- 58.Pothuaud L, Carceller P, Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone. 2008;42(4):775–87. doi: 10.1016/j.bone.2007.11.018. doi:10.1016/ j.bone.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 59•.Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29(3):518–30. doi: 10.1002/jbmr.2176. doi:10.1002/jbmr.2176. [In-depth review article on the current body of knowledge with respect to the Trabecular Bone Score, provides also nice illustrations.] [DOI] [PubMed] [Google Scholar]
- 60.Bousson V, Bergot C, Sutter B, Levitz P, Cortet B. Trabecular bone score (TBS): available knowledge, clinical relevance, and future prospects. Osteoporos Int. 2012;23(5):1489–501. doi: 10.1007/s00198-011-1824-6. doi:10. 1007/s00198-011-1824-6. [DOI] [PubMed] [Google Scholar]
- 61.Winzenrieth R, Dufour R, Pothuaud L, Hans D. A retrospective case-control study assessing the role of trabecular bone score in postmenopausal Caucasian women with osteopenia: analyzing the odds of vertebral fracture. Calcif Tissue Int. 2010;86(2):104–9. doi: 10.1007/s00223-009-9322-y. doi:10.1007/s00223-009-9322-y. [DOI] [PubMed] [Google Scholar]
- 62.Pothuaud L, Barthe N, Krieg MA, Mehsen N, Carceller P, Hans D. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case-control study. J Clin Densitom. 2009;12(2):170–6. doi: 10.1016/j.jocd.2008.11.006. doi:10.1016/j.jocd.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Rabier B, Heraud A, Grand-Lenoir C, Winzenrieth R, Hans D. A multicentre, retrospective case-control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): analysing the odds of vertebral fracture. Bone. 2010;46(1):176–81. doi: 10.1016/j.bone.2009.06.032. doi:10.1016/j.bone.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 64.Del Rio LM, Winzenrieth R, Cormier C, Di Gregorio S. Is bone microarchitecture status of the lumbar spine assessed by TBS related to femoral neck fracture? A Spanish case-control study. Osteoporos Int. 2013;24(3):991–8. doi: 10.1007/s00198-012-2008-8. doi:10.1007/s00198-012-2008-8. [DOI] [PubMed] [Google Scholar]
- 65.Vasic J, Petranova T, Povoroznyuk V, Barbu CG, Karadzic M, Gojkovic F, et al. Evaluating spine micro-architectural texture (via TBS) discriminates major osteoporotic fractures from controls both as well as and independent of site matched BMD: the Eastern European TBS study. J Bone Miner Metab. 2014;32(5):556–62. doi: 10.1007/s00774-013-0529-7. doi:10.1007/s00774-013-0529-7. [DOI] [PubMed] [Google Scholar]
- 66.Leib E, Winzenrieth R, Lamy O, Hans D. Comparing bone microarchitecture by trabecular bone score (TBS) in Caucasian American women with and without osteoporotic fractures. Calcif Tissue Int. 2014;95(3):201–8. doi: 10.1007/s00223-014-9882-3. doi:10.1007/s00223-014-9882-3. [DOI] [PubMed] [Google Scholar]
- 67.Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2762–9. doi: 10.1002/jbmr.499. doi:10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 68.Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int. 2013;24(1):77–85. doi: 10.1007/s00198-012-2188-2. doi:10.1007/s00198-012-2188-2. [DOI] [PubMed] [Google Scholar]
- 69.Leib E, Winzenrieth R, Aubry-Rozier B, Hans D. Vertebral microarchitecture and fragility fracture in men: a TBS study. Bone. 2014;62:51–5. doi: 10.1016/j.bone.2013.12.015. doi:10.1016/j.bone.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 70.Leslie WD, Aubry-Rozier B, Lix LM, Morin SN, Majumdar SR, Hans D. Spine bone texture assessed by trabecular bone score (TBS) predicts osteoporotic fractures in men: the Manitoba Bone Density Program. Bone. 2014;67:10–4. doi: 10.1016/j.bone.2014.06.034. doi:10.1016/j.bone.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 71.Schousboe JT, Vo T, Taylor BC, Cawthon PM, Schwartz AV, Bauer DC, et al. Prediction of Incident Major Osteoporotic and Hip Fractures by Trabecular Bone Score (TBS) and Prevalent Radiographic Vertebral Fracture in Older Men. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2713. doi:10.1002/jbmr.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCloskey EV, Oden A, Harvey NC, Leslie WD, Hans D, Johansson H, et al. Adjusting fracture probability by trabecular bone score. Calcif Tissue Int. 2015;96(6):500–9. doi: 10.1007/s00223-015-9980-x. doi:10.1007/s00223-015-9980-x. [DOI] [PubMed] [Google Scholar]
- 73.McCloskey EV, Oden A, Harvey NC, Leslie WD, Hans D, Johansson H, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2734. doi:10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]
- 74.Bousson V, Bergot C, Sutter B, Thomas T, Bendavid S, Benhamou CL, et al. Trabecular bone score: where are we now? Joint Bone Spine. 2015;82(5):320–5. doi: 10.1016/j.jbspin.2015.02.005. doi:10.1016/j.jbspin.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 2: trabecular bone score. J Clin Densitom. 2015;18(3):309–30. doi: 10.1016/j.jocd.2015.06.008. doi:10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Winzenrieth R, Michelet F, Hans D. Three-dimensional (3D) microarchitecture correlations with 2D projection image gray-level variations assessed by trabecular bone score using high-resolution computed tomographic acquisitions: effects of resolution and noise. J Clin Densitom. 2013;16(3):287–96. doi: 10.1016/j.jocd.2012.05.001. doi:10.1016/j.jocd.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom. 2011;14(3):302–12. doi: 10.1016/j.jocd.2011.05.005. doi:10.1016/j.jocd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Roux JP, Wegrzyn J, Boutroy S, Bouxsein ML, Hans D, Chapurlat R. The predictive value of trabecular bone score (TBS) on whole lumbar vertebrae mechanics: an ex vivo study. Osteoporos Int. 2013;24(9):2455–60. doi: 10.1007/s00198-013-2316-7. doi:10.1007/s00198-013-2316-7. [DOI] [PubMed] [Google Scholar]
- 79.Popp AW, Buffat H, Eberli U, Lippuner K, Ernst M, Richards RG, et al. Microstructural parameters of bone evaluated using HR-pQCT correlate with the DXA-derived cortical index and the trabecular bone score in a cohort of randomly selected premenopausal women. PLoS One. 2014;9(2):e88946. doi: 10.1371/journal.pone.0088946. doi:10.1371/journal.pone.0088946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva BC, Boutroy S, Zhang C, McMahon DJ, Zhou B, Wang J, et al. Trabecular bone score (TBS)–a novel method to evaluate bone microarchitectural texture in patients with primary hyper-parathyroidism. J Clin Endocrinol Metab. 2013;98(5):1963–70. doi: 10.1210/jc.2012-4255. doi:10.1210/jc.2012-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silva BC, Walker MD, Abraham A, Boutroy S, Zhang C, McMahon DJ, et al. Trabecular bone score is associated with volumetric bone density and microarchitecture as assessed by central QCT and HRpQCT in Chinese American and white women. J Clin Densitom. 2013;16(4):554–61. doi: 10.1016/j.jocd.2013.07.001. doi:10.1016/j.jocd.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amstrup AK, Jakobsen NF, Moser E, Sikjaer T, Mosekilde L, Rejnmark L. Association between bone indices assessed by DXA, HR-pQCT and QCT scans in post-menopausal women. J Bone Miner Metab. 2015 doi: 10.1007/s00774-015-0708-9. doi:10.1007/s00774-015-0708-9. [DOI] [PubMed] [Google Scholar]
- 83.Bandirali M, Poloni A, Sconfienza LM, Messina C, Papini GD, Petrini M, et al. Short-term precision assessment of trabecular bone score and bone mineral density using dual-energy X-ray absorptiometry with different scan modes: an in vivo study. Eur Radiol. 2015;25(7):2194–8. doi: 10.1007/s00330-015-3606-6. doi:10.1007/s00330-015-3606-6. [DOI] [PubMed] [Google Scholar]
- 84.Krueger D, Libber J, Binkley N. Spine trabecular bone score precision, a comparison between GE lunar standard and high-resolution densitometers. J Clin Densitom. 2015;18(2):226–32. doi: 10.1016/j.jocd.2014.11.003. doi:10.1016/j.jocd.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 85.Davies KM, Stegman MR, Heaney RP, Recker RR. Prevalence and severity of vertebral fracture: the Saunders county bone quality study. Osteoporos Int. 1996;6(2):160–5. doi: 10.1007/BF01623941. [DOI] [PubMed] [Google Scholar]
- 86.O'Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ. The prevalence of vertebral deformity in european men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res. 1996;11(7):1010–8. doi: 10.1002/jbmr.5650110719. doi:10.1002/jbmr. 5650110719. [DOI] [PubMed] [Google Scholar]
- 87.Siris ES, Genant HK, Laster AJ, Chen P, Misurski DA, Krege JH. Enhanced prediction of fracture risk combining vertebral fracture status and BMD. Osteoporos Int. 2007;18(6):761–70. doi: 10.1007/s00198-006-0306-8. doi:10.1007/s00198-006-0306-8. [DOI] [PubMed] [Google Scholar]
- 88.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285(3):320–3. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 89.Burger H, van Daele PL, Algra D, Hofman A, Grobbee DE, Schutte HE, et al. Vertebral deformities as predictors of nonvertebral fractures. BMJ. 1994;309(6960):991–2. doi: 10.1136/bmj.309.6960.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ., 3rd Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7(2):221–7. doi: 10.1002/jbmr.5650070214. doi:10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 91.Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of vertebral fracture in a clinical setting. Osteoporos Int. 2000;11(7):577–82. doi: 10.1007/s001980070078. doi:10.1007/s001980070078. [DOI] [PubMed] [Google Scholar]
- 92.Bartalena T, Giannelli G, Rinaldi MF, Rimondi E, Rinaldi G, Sverzellati N, et al. Prevalence of thoracolumbar vertebral fractures on multidetector CT: underreporting by radiologists. Eur J Radiol. 2009;69(3):555–9. doi: 10.1016/j.ejrad.2007.11.036. doi:10.1016/j.ejrad.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 93.Link TM. Radiology of osteoporosis. Can Assoc Radiol J. 2015 doi: 10.1016/j.carj.2015.02.002. doi:10.1016/j.carj.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 94••.Griffith JF. Identifying osteoporotic vertebral fracture. Quant Imaging Med Surg. 2015;5(4):592–602. doi: 10.3978/j.issn.2223-4292.2015.08.01. doi:10.3978/j.issn.2223-4292.2015.08.01. [Very good teaching article on how to identify osteoporotic vertebral fractures and how to differentiate a vertebral fracture from other physiological and pathological vertrebal changes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95••.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–48. doi: 10.1002/jbmr.5650080915. [First description of this universal and famous scoring system of vertebral fractures as developed by Dr. Genant. It provides a detailed explanation and depiction on how to diagnose a vertebral fracture.] [DOI] [PubMed] [Google Scholar]
- 96.Genant HK, Delmas PD, Chen P, Jiang Y, Eriksen EF, Dalsky GP, et al. Severity of vertebral fracture reflects deterioration of bone microarchitecture. Osteoporos Int. 2007;18(1):69–76. doi: 10.1007/s00198-006-0199-6. doi:10.1007/s00198-006-0199-6. [DOI] [PubMed] [Google Scholar]
- 97.Siris E, Adachi JD, Lu Y, Fuerst T, Crans GG, Wong M, et al. Effects of raloxifene on fracture severity in postmenopausal women with osteoporosis: results from the MORE study. Multiple outcomes of raloxifene evaluation. Osteoporos Int. 2002;13(11):907–13. doi: 10.1007/s001980200125. doi:10.1007/s001980200125. [DOI] [PubMed] [Google Scholar]
- 98.Vokes T, Bachman D, Baim S, Binkley N, Broy S, Ferrar L, et al. Vertebral fracture assessment: the 2005 ISCD Official Positions. J Clin Densitom. 2006;9(1):37–46. doi: 10.1016/j.jocd.2006.05.006. doi:10.1016/j.jocd.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 99.Adams JE. Advances in bone imaging for osteoporosis. Nat Rev. 2013;9(1):28–42. doi: 10.1038/nrendo.2012.217. doi:10.1038/nrendo.2012.217. [DOI] [PubMed] [Google Scholar]
- 100.Hospers IC, van der Laan JG, Zeebregts CJ, Nieboer P, Wolf-fenbuttel BH, Dierckx RA, et al. Vertebral fracture assessment in supine position: comparison by using conventional semi-quantitative radiography and visual radiography. Radiology. 2009;251(3):822–8. doi: 10.1148/radiol.2513080887. doi:10.1148/radiol.2513080887. [DOI] [PubMed] [Google Scholar]
- 101.Lewiecki EM, Laster AJ. Clinical review: Clinical applications of vertebral fracture assessment by dual-energy X-ray absorptiometry. J Clin Endocrinol Metab. 2006;91(11):4215–22. doi: 10.1210/jc.2006-1178. doi:10.1210/jc.2006-1178. [DOI] [PubMed] [Google Scholar]
- 102.Olenginski TP, Newman ED, Hummel JL, Hummer M. Development and evaluation of a vertebral fracture assessment program using IVA and its integration with mobile DXA. J Clin Densitom. 2006;9(1):72–7. doi: 10.1016/j.jocd.2005.08.002. doi:10.1016/j.jocd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Greenspan SL, von Stetten E, Emond SK, Jones L, Parker RA. Instant vertebral assessment: a noninvasive dual X-ray absorptiometry technique to avoid misclassification and clinical mismanagement of osteoporosis. J Clin Densitom. 2001;4(4):373–80. doi: 10.1385/jcd:4:4:373. [DOI] [PubMed] [Google Scholar]
- 104.Jager PL, Jonkman S, Koolhaas W, Stiekema A, Wolffenbuttel BH, Slart RH. Combined vertebral fracture assessment and bone mineral density measurement: a new standard in the diagnosis of osteoporosis in academic populations. Osteoporos Int. 2011;22(4):1059–68. doi: 10.1007/s00198-010-1293-3. doi:10.1007/s00198-010-1293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schousboe JT, Ensrud KE, Nyman JA, Kane RL, Melton LJ., 3rd Cost-effectiveness of vertebral fracture assessment to detect prevalent vertebral deformity and select postmenopausal women with a femoral neck T-score [-2.5 for alendronate therapy: a modeling study. J Clin Densitom. 2006;9(2):133–43. doi: 10.1016/j.jocd.2005.11.004. doi:10. 1016/j.jocd.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 106•.Rosen HN, Vokes TJ, Malabanan AO, Deal CL, Alele JD, Olenginski TP, et al. The official positions of the international society for clinical densitometry: vertebral fracture assessment. J Clin Densitom. 2013;16(4):482–8. doi: 10.1016/j.jocd.2013.08.003. doi:10.1016/j.jocd.2013.08.003. [Article summarizing the most recent advances in VFA.] [DOI] [PubMed] [Google Scholar]
- 107.Ismail AA, Cooper C, Felsenberg D, Varlow J, Kanis JA, Silman AJ, European Vertebral Osteoporosis Study Group et al. Number and type of vertebral deformities: epidemiological characteristics and relation to back pain and height loss. Osteoporos Int. 1999;9(3):206–13. doi: 10.1007/s001980050138. [DOI] [PubMed] [Google Scholar]
- 108.Vogt TM, Ross PD, Palermo L, Musliner T, Genant HK, Black D, Fracture Intervention Trial Research Group et al. Vertebral fracture prevalence among women screened for the fracture intervention trial and a simple clinical tool to screen for undiag-nosed vertebral fractures. Mayo Clin Proc. 2000;75(9):888–96. doi: 10.4065/75.9.888. [DOI] [PubMed] [Google Scholar]
- 109.Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton IL, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893–9. doi: 10.1359/JBMR.040134. doi:10.1359/jbmr.040134. [DOI] [PubMed] [Google Scholar]
- 110.Faulkner KG, Cummings SR, Black D, Palermo L, Gluer CC, Genant HK. Simple measurement of femoral geometry predicts hip fracture: the study of osteoporotic fractures. J Bone Miner Res. 1993;8(10):1211–7. doi: 10.1002/jbmr.5650081008. doi:10.1002/jbmr.5650081008. [DOI] [PubMed] [Google Scholar]
- 111.Ripamonti C, Lisi L, Avella M. Femoral neck shaft angle width is associated with hip-fracture risk in males but not independently of femoral neck bone density. Br J Radiol. 2014;1037(87):20130358. doi: 10.1259/bjr.20130358. doi:10.1259/bjr.20130358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beck TJ, Ruff CB, Warden KE, Scott WW, Jr, Rao GU. Predicting femoral neck strength from bone mineral data. A structural approach. Investig Radiol. 1990;25(1):6–18. doi: 10.1097/00004424-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 113.Leslie WD, Pahlavan PS, Tsang JF, Lix LM. Prediction of hip and other osteoporotic fractures from hip geometry in a large clinical cohort. Osteoporos Int. 2009;20(10):1767–74. doi: 10.1007/s00198-009-0874-5. doi:10. 1007/s00198-009-0874-5. [DOI] [PubMed] [Google Scholar]
- 114.Gnudi S, Malavolta N, Testi D, Viceconti M. Differences in proximal femur geometry distinguish vertebral from femoral neck fractures in osteoporotic women. Br J Radiol. 2004;77(915):219–23. doi: 10.1259/bjr/79551075. doi:10.1259/bjr/79551075. [DOI] [PubMed] [Google Scholar]
- 115.Leslie WD, Lix LM, Morin SN, Johansson H, Oden A, McCloskey EV, et al. Hip axis length is a FRAX- and bone density-independent risk factor for hip fracture in women. J Clin Endocrinol Metab. 2015;100(5):2063–70. doi: 10.1210/jc.2014-4390. doi:10.1210/jc.2014-4390. [DOI] [PubMed] [Google Scholar]
- 116.Broy SB, Cauley JA, Lewiecki ME, Schousboe JT, Shepherd JA, Leslie WD. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 1: hip geometry. J Clin Densitom. 2015;18(3):287–308. doi: 10.1016/j.jocd.2015.06.005. doi:10.1016/j.jocd.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 117.Leslie WD, Lix LM, Morin SN, Johansson H, Oden A, McCloskey EV, et al. Adjusting hip fracture probability in men and women using hip axis length: the manitoba bone density database. J Clin Densitom. 2015 doi: 10.1016/j.jocd.2015.07.004. doi:10.1016/j.jocd.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 118.Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23(12):1892–904. doi: 10.1359/JBMR.080802. doi:10.1359/jbmr.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alonso CG, Curiel MD, Carranza FH, Cano RP, Perez AD. Femoral bone mineral density, neck-shaft angle and mean femoral neck width as predictors of hip fracture in men and women. Multicenter Project for Research in Osteoporosis. Osteoporos Int. 2000;11(8):714–20. [PubMed] [Google Scholar]
- 120.Rivadeneira F, Zillikens MC, De Laet CE, Hofman A, Uitter-linden AG, Beck TJ, et al. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. J Bone Miner Res. 2007;22(11):1781–90. doi: 10.1359/jbmr.070712. doi:10. 1359/jbmr.070712. [DOI] [PubMed] [Google Scholar]
- 121.Tenne M, McGuigan FE, Ahlborg H, Gerdhem P, Akesson K. Variation in the PTH gene, hip fracture, and femoral neck geometry in elderly women. Calcif Tissue Int. 2010;86(5):359–66. doi: 10.1007/s00223-010-9351-6. doi:10.1007/s00223-010-9351-6. [DOI] [PubMed] [Google Scholar]