ABSTRACT
We report the case of a cord blood haematopoietic stem cell transplant recipient who was vaccinated for Yellow Fever (YF) 7 days before initiating chemotherapy and had persistent YF antibodies more than 3 years after vaccination. Since the stem cell donor was never exposed to wild YF or to the YF vaccine, and our patient was not exposed to YF or revaccinated, this finding strongly suggests the persistence of recipient immunity. We briefly discuss potential consequences of incomplete elimination of recipient's leukocytes following existing haematopoietic cancer treatments.
Keywords: cord blood stem cell transplant, immunosuppression, vaccine, yellow fever
Introduction
Yellow fever (YF) is a mosquito-borne disease, endemic in several countries in Latin America and Africa with approximately 5000 cases reported annually, but the real incidence is believed to be up to 10-50 fold higher. No antiviral treatment is available,1 and the live attenuated YF vaccine has been used for several decades as the main strategy for prevention of the disease.2 YF vaccine is not recommended for individuals with impaired immunity such as stem cell transplant recipients due to the risk of severe adverse events associated with YF vaccine virus replication.3
Patient presentation
We previously reported the case of a 39 year-old male patient with acute myeloid leukemia (AML) who started chemotherapy in February, 2011, 7 days after receiving a first dose of the live attenuated 17DD substrain YF vaccine (102 VFA007L lot, Bio-Manguinhos, RJ, Brazil).4 The patient resided in an area where YF vaccine was not routinely recommended and received his first YF vaccine dose due to a planned visit to Central America, where the vaccine certificate is required for travelers disembarking from YF-endemic countries. He presented a prolonged, asymptomatic viremia due to the vaccine virus as monitored by Real-Time Polymerase Chain Reaction (RT-PCR), for 15 days after vaccination. The detection of viremia by the YF vaccine virus reinforces the assumption that this was indeed his first lifetime exposure to the YF virus.5,6 Neutralizing antibodies (NA) against 17DD were measured on day 28 after vaccination, indicating protective NA levels of 3,103 mIU/ml.7
The patient was followed after successful haematopoietic stem cell transplantation (HSCT) using double cord blood stem cells, with 100% chimerism from a single cord donor, at a chimerism test sensitivity of 5%. He received a conditioning treatment with cyclophosphamide (4,750 mg on day −6), fludarabine (66.3 mg daily on days −6 to −2), thiotepa (475 mg daily on days −5 and −4) and total body irradiation (200 cGy daily on days −2 and −1). The transplantation was performed 6 months after the initial diagnosis of AML and YF vaccination, and both transplanted cords were donated from infants born in France, a YF-free area. He remained free of disease recurrence up to the end of 2014.
More than 3 years after the vaccination and initial chemotherapy (Fig. 1), a new YF-specific NA measurement revealed that the patient maintained antibody titers of 2,322 mIU/ml, very similar to the initial levels measured 28 days after vaccination, and above the 794 mIU/ml seropositive threshold according to the laboratory reference.8
Figure 1.
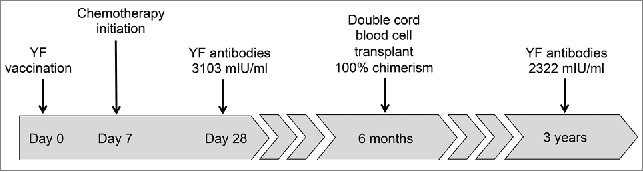
Timeline of treatment and Yellow Fever antibodies measurement after YF vaccination.
Discussion
While residual immunity against vaccine antigens has been commonly observed after HSCT,9-11 the source of immune reacting cells - whether donor or recipient-derived - cannot be unequivocally determined in virtually any case. However, in this report, the patient had never been exposed to YF before vaccination, and after the stem cell transplantation he was not revaccinated against YF nor did he visit YF-endemic areas. Furthermore, since stem cell donors were born in France and YF vaccine is contra-indicated for infants under 6 months, we can confidently assume that donors were never exposed to wild YF or to the YF vaccine. Unfortunately, we do not have information on vaccination status of the cord blood donors' mothers. However, cord blood grafts are not expected to contain YF-reactive memory B cells from the cord blood donors' mothers; instead, had the mothers been vaccinated against YF, we could expect transplacental transfer of YF-specific antibodies, which would likely last for up to 6 months after transplantation. Our patient maintained antibody titers for more than 30 months after transplantation. This finding suggests the persistence of recipient immunity, potentially due to the existence of functionally active residual memory B-lymphocytes.
Sterilizing elimination of recipient leucocytes is likely not achieved despite modern conditioning regimens and total body irradiation. The maintenance of recipient's antibody response against YF virus may indicate that a benign recall adaptive immunity is preserved. Preservation of adaptive immunity has practical implications, particularly against infectious agents for which only live attenuated vaccines are available. This is the case for YF, varicella, measles and rotavirus. Since the minimal waiting period before a HSCT recipient can safely receive a live attenuated vaccine is 2 years,3 persistence of NA may confer at least partial protection while revaccination is not feasible.
However, the recurrence of malignant clonal cells is the most undesirable consequence of such incomplete cellular eradication. Current chemotherapy erratically eliminates both malignant and benign cells, and does not guarantee sterilizing cancer elimination. Yet, in the context of HSCT, incomplete removal of benign cells may provide a source of beneficial graft-versus-tumor stimulus.
A concurrent explanation for the persistence of YF-specific antibodies in this case would be the stimulation of B cells derived from the donated stem cells in the event of residual persisting viremia by the YF vaccine virus. This event would be unlikely in the presence of neutralizing antibodies, which were detected at 28 days after vaccination. However, we cannot exclude that stable proteins derived from YF vaccine virus could have stimulated humoral responses detected at 3 years after vaccination.
While strategies for sterilizing elimination of specific cellular targets with simultaneous preservation of benign or protective cells are unavailable, a deep understanding of the complex mechanisms through which the immune system may respond to available therapies is crucial and may provide insightful clues for the advancement of modern applied immunology research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge Simone Ojima for contributing with patient information, and Jessica Ramos for suggestions on the manuscript.
Authors' contributions
VAS and EGK contributed with manuscript concept design and initial draft; MSF and ECS performed laboratory analysis; CAR, VR and YSN contributed with clinical data acquisition and interpretation. All coauthors critically reviewed and approved the final manuscript.
References
- [1].Monath TP. Yellow fever: an update. Lancet Infect Dis 2001; 1:11-20; PMID:11871403; http://dx.doi.org/ 10.1016/S1473-3099(01)00016-0 [DOI] [PubMed] [Google Scholar]
- [2].Monath TP. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines: Saunders, 2008:1748 [Google Scholar]
- [3].Ljungman P, Cordonnier C, Einsele H, Englund J, Machado CM, Storek J, Small T. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplantation 2009; 44:521-6; PMID:19861986; http://dx.doi.org/ 10.1038/bmt.2009.263 [DOI] [PubMed] [Google Scholar]
- [4].Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, Huebner RE, Janoff EN, Justice AC, Kuritzkes D, et al.. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis 2008; 47:542-53; PMID:18627268; http://dx.doi.org/ 10.1086/590150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reinhardt B, Jaspert R, Niedrig M, Kostner C, L'Age-Stehr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol 1998; 56:159-67; PMID:9746073; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- [6].Barrett AD, Teuwen DE. Yellow fever vaccine - how does it work and why do rare cases of serious adverse events take place? Curr Opin Immunol 2009; 21:308-13; PMID:19520559; http://dx.doi.org/ 10.1016/j.coi.2009.05.018 [DOI] [PubMed] [Google Scholar]
- [7].Avelino-Silva VI, Leal FE, Sabino EC, Nishiya AS, da Silva. Freire M, Blumm F, Rocha V, Rodrigues CA, Novis YS, et al.. Yellow fever vaccine viremia following ablative BM suppression in AML. Bone Marrow Transplantation 2013; 48:1008-9; PMID:23334273; http://dx.doi.org/ 10.1038/bmt.2012.277 [DOI] [PubMed] [Google Scholar]
- [8].Simoes M, Camacho LA, Yamamura AM, Miranda EH, Cajaraville AC, da Silva. Freire M. Evaluation of accuracy and reliability of the plaque reduction neutralization test (micro-PRNT) in detection of yellow fever virus antibodies. Biologicals 2012; 40:399-404; PMID:23034357; http://dx.doi.org/ 10.1016/j.biologicals.2012.09.005 [DOI] [PubMed] [Google Scholar]
- [9.].Spoulou V, Giannaki M, Vounatsou M, Bakoula C, Grafakos S. Long-term immunity to measles, mumps and rubella after MMR vaccination among children with bone marrow transplants. Bone Marrow Transplant 2004; 33:1187-90; PMID:15077129; http://dx.doi.org/ 10.1038/sj.bmt.1704476 [DOI] [PubMed] [Google Scholar]
- [10].Parkkali T, Ruutu T, Stenvik M, Kuronen T, Kayhty H, Hovi T, Olander RM, Volin L, Ruutu P. Loss of protective immunity to polio, diphtheria and Haemophilus influenzae type b after allogeneic bone marrow transplantation. APMIS 1996; 104:383-8; PMID:8703445; http://dx.doi.org/ 10.1111/j.1699-0463.1996.tb00731.x [DOI] [PubMed] [Google Scholar]
- [11].Parkkali T, Olander RM, Ruutu T, Vuontela K, Volin L, Eskola J, Ruutu P. A randomized comparison between early and late vaccination with tetanus toxoid vaccine after allogeneic BMT. Bone Marrow Transplantation 1997; 19:933-8; PMID:9156269; http://dx.doi.org/ 10.1038/sj.bmt.1700768 [DOI] [PubMed] [Google Scholar]


