Fig. 2.
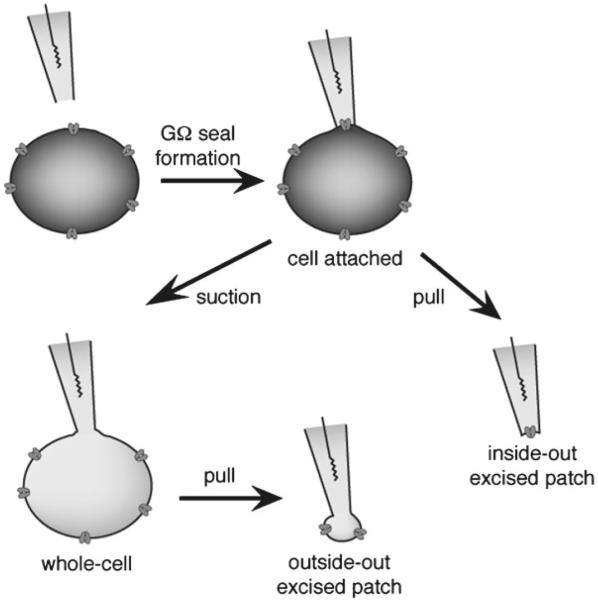
Different patch-clamp configurations. In a first step, the patch pipette is moved to the surface of the cell membrane and suction is applied to achieve a GΩ seal between the glass pipette and the membrane. This patch configuration is called cell-attached and is used to monitor very high current resolution through single channels. The cell content is intact, making it tricky to determine which ions contribute to the current since the ion concentrations inside the cell are unknown. Cell-attached is also an intermediate step towards getting whole-cell or excised-patch configurations. Inside-out, excised-patch configuration is achieved by pulling the pipette away from the cell. A small piece of membrane will stay attached to the pipette, with the cytosolic part facing the bath solution, allowing perfusion of drugs that normally have a cytosolic mode of action. Whole-cell recording is achieved from cell-attached mode after giving gentle suction to break the piece of membrane at the end of the tip. It is used to measure the average current through the entire cell while controlling ion concentrations on both sides of the membrane. Outside-out, excised patch is obtained from whole-cell recording by pulling the pipette away from the cell. The cell membrane will break and will reform as a convex bleb, leaving a little piece of membrane at the end of the pipette tip, with the external side facing the bath. Single-channel recording can be achieved, with the advantage of having control over ionic concentrations of both sides of the membrane. For electrophysiological characterization of Kv channels photochromic blockers, whole-cell and inside-out configurations have been used