ABSTRACT
Persistence of bactericidal antibodies following vaccination is extremely important for protection against invasive meningococcal disease, given the epidemiology and rapid progression of meningococcal infection. We present an analysis of antibody persistence and booster response to MenACWY-CRM, in adolescents, children and infants, from 7 clinical studies. Immunogenicity was assessed using the serum bactericidal assay with both human and rabbit complement. Post-vaccination hSBA titers were high, with an age- and serogroup-specific decline in titers up to 1 y and stable levels up to 5 y The waning of hSBA titers over time was more pronounced among infants and toddlers and the greatest for serogroup A. However, rSBA titers against serogroup A were consistently higher and showed little decline over time, suggesting that protection against this serogroup may be sustained. A single booster dose of MenACWY-CRM administered at 3 to 5 y induced a robust immune response in all age groups.
KEYWORDS: booster, hSBA, MenACWY-CRM, meningococcal, persistence, rSBA
Introduction
Invasive meningococcal disease (IMD) is a rare but serious disease with case fatality rates ranging between 10–15%.1 One in 5 survivors of IMD suffer permanent sequelae including hearing loss, neurological impairment, seizures and intellectual disabilities, all of which can seriously impact quality of life.1
Although, in general, the highest incidence of IMD occurs in infants below the age of 12 months most countries report a second incidence peak in individuals between the ages of 15 and 19 years, in whom transmission is facilitated by close living conditions and sociobehavioral factors. Asymptomatic carriage of potentially virulent meningococcal disease strains also peaks in this age group.2 The incidence of meningococcal disease also varies by geographical location, time of year, and serogroup, with most cases of IMD being caused by serogroups A, B, C, W and Y.
Three quadrivalent conjugate meningococcal vaccines (MenACWY) are currently licensed worldwide and included in the national immunization programs of certain countries. Recommendations, in particular, by the US Advisory Committee on Immunization Practices (ACIP) include routine vaccination with MenACWY for adolescents at 11 y of age with a booster dose administered 5 y later.3 The UK Department of Health recommends a catch up MenACWY vaccination program for all 13–19 -year-olds and first-time university students up to the age of 25.4 Routine MenACWY vaccination was recently recommended for infants, starting at 2 months of age in Argentina5 and starting at 9 months of age in Saudi Arabia.6
The quadrivalent meningococcal CRM197-conjugate vaccine MenACWY-CRM (Menveo®, GlaxoSmithKline Vaccines Srl, Siena, Italy; formerly Novartis Vaccines) is licensed in over 60 countries worldwide for use in individuals as young as 2 y of age. In the United States, Canada, Argentina, Korea and a few other countries, MenACWY-CRM has been approved for use in infants from 2 months of age. In clinical studies, MenACWY-CRM has been found to have acceptable safety and immunogenicity profiles in all indicated age groups.7-10
This review appraises data from 7 phase 3 and phase 4 studies, with the objective of providing an overview of antibody persistence following primary vaccination with MenACWY-CRM vaccine, and responses to booster doses of the vaccine.9,10,11-19 Only data pertaining to the recommended dose schedules of MenACWY-CRM in each age group is assessed here : a single dose in children 2–10 y of age, adolescents and adults; 4 doses at 2, 4, 6 and 12 months of age in infants; and 2 doses given to unvaccinated children between 7–23 months of age. All the studies included in this review were conducted in accordance with good clinical practice and International Conference on Harmonisation of Techniques for Requirements for Registration of Pharmaceuticals for Human Use guidelines, and were approved by Institutional Review Boards (IRBs) or Ethics Committees (ECs) in each country, as appropriate, prior to start of the study. The design and methodology of these studies are summarized in Table 1.
Table 1.
Clinical studies assessing antibody persistence and booster response following MenACWY-CRM vaccination in adolescents, children and infants.
| Study number identifier*) | Population | Na | Country | Study Design | Assays used | Persistence/Booster Assessments | Key Results | Manuscript reference |
|---|---|---|---|---|---|---|---|---|
| Study 1 (NCT00262041) | Adolescents (11–17 years) | 524 | USA | A Phase 2, Randomized, Single-blind, Controlled, Multicenter Study to Compare the Safety and Immune Response of One Dose of Novartis Meningococcal ACWY Conjugate Vaccine With the Safety and Immune Response of One Dose of Licensed Meningococcal ACWY Polysaccharide Vaccine Administered to Healthy Adolescents 11 to 17 Years of Age | hSBA, rSBA | Persistence: 12 months after 1-dose primary vaccination | • Single dose of CRM-conjugated MenACWY well-tolerated and immunogenic with persistence of antibodies for at least 12 months• Immune response to conjugated MenACWY significantly higher than to polysaccharide comparator, across serogroups | Jackson et al. Pediatric Infec Dis J2009; 28:86–91 |
| Study 2 (NCT01682876) | Children (2–10 years) | 715 | USA | A Phase 3b, Randomized, Observer-Blind, Placebo-Controlled Multi-Center Study Comparing Immunogenicity, Safety and 1 Year Persistence of Antibodies After Either One or Two Doses of Novartis Meningococcal ACWY Conjugate Vaccine, Administered to Healthy Children 2 to 10 Years of Age | hSBA | Persistence: 12 months after 1-dose primary vaccination | • Both 1- and 2-dose series are well-tolerated and immunogenic in children aged 2–10 y• 2-dose series induces higher antibody titers immediately post vaccination but differences are limited up to 1 y• Both schedules induce good antibody persistence at 1 y | Johnston et al.Pediatric Infec Dis J2015; In Press |
| Study 3 (NCT00856297) | Adolescents and adults (11–18 y at time of enrolment in parent study) | 389 | USA | An Open-Label, Multi-Center Study to Evaluate the Persistence Of Antibody Responses Among Adolescents Who Previously Received Novartis MenACWY Conjugate Vaccine or Commercially Available MenACWY Conjugate Vaccine | hSBA, rSBA | Persistence: 21 months, 3 y and 5 y after 1-dose primary vaccination Booster: 3 y after primary vaccination | • Antibody titers decline by 21 months after vaccination and remain relatively stable by 3 and 5 y after vaccination, after either a single dose of MenACWY-CRM or Menactra• Persistence robust for serogroups W, Y and to a lesser extent C. Low for serogroup A• Booster dose of MenACWY-CRM at 3 y is well-tolerated and induces a robust anamnestic immune response, irrespective of priming vaccine• No long-term safety concerns identified | Gill et al. Human Vaccines2010 ;6 (11) :881–887Baxter et al. J Pediatr 2014 ; 164: 1409–1415Baxter et al. Pediatric Infec Dis J2014; 33: 1169–1176 |
| Study 4 (NCT01823536) | Children (7–15 years) | 465 | USA | A Phase IV, Open-label, Controlled, Multi-center Study to Evaluate the 5-year Antibody Persistence Among Children Who Previously Received Novartis MenACWY Conjugate Vaccine at 2 to 10 Years of Age and to Assess the Immune Response to a Single Dose of Novartis MenACWY Conjugate Vaccine | hSBA | Persistence: 5 y after 1- and 2-dose primary vaccination Booster: 5 y after primary vaccination | • Good persistence up to 5 y after vaccination for serogroups C W and Y• Little to no difference in persistence of antibodies at 5 y with either 1 or 2 priming doses• Booster at 5 y well-tolerated and induces robust immune response across serogroups | Block et al.Vaccine201533: 2175–2182 |
| Study 5 (NCT00667602) | Infants (6–8 months) and toddlers (12 months) | 662 | Germany Australia | A Phase 3, Open-Label, Randomized, Multi-Center Study to Evaluate the Safety and Immunogenicity After One or Two Doses of Novartis Meningococcal ACWY Conjugate Vaccine Administered to Healthy Infants and Toddlers | hSBA | Persistence: 7 months after 2-dose primary series | • 2 doses of MenACWY in toddlers induced a substantial immune response against all 4 serogroups• A single dose of MenACWY also induced a robust response, although lower than a MenC conjugate vaccine (against serogroup C)• Substantial persistence seen at ˜7 months after 2 doses across serogroups• Concomitant administration with Infanrix-Hexa and Prevnar supported• Both doses well-tolerated, with no major safety concerns | Data on file |
| Study 6 (NCT00474526) | Infants (2 months) | 479 (US) | USA, Latin America | A Phase 3, Open-Label, Randomized, Parallel-Group, Multi-Center Study to Evaluate the Safety and Immunogenicity of Novartis Meningococcal ACWY Conjugate Vaccine When Administered With Routine Infant Vaccinations to Healthy Infants | hSBA | Persistence: 6 months after 3-dose infant series | • MenACWY highly immunogenic in infants and toddlers• 4 doses in infants and 2 doses in toddlers well-tolerated with no major safety concerns• MenACWY can be safely co-administered with routine infant and toddler vaccinations | Klein et al.Pediatr Infect Dis J 2012; 31: 64–71Tregnaghi et al.Int J Infect Dis 2014; 26: 22-e30 |
| Study 7 (NCT01148017) | Children (40 and 60 months of age) | 433 | USA | A Phase IIIb, Open-Label, Controlled, Multi-Center Study to Evaluate the Persistence Of Antibody Responses Among Children Who Previously Received Novartis MenACWY Conjugate Vaccine | hSBA, rSBA | Persistence: 40 months and 60 months after 4-dose infant series and 2-dose toddler series Booster: 60 months of age | • Modest persistence up to 5 y after vaccination as infants or toddlers for A and C; high persistence for W and Y• Higher persistence at 40 and 60 months of age after 2-dose toddler series than after 4-dose infant series• Booster dose at 60 months of age resulted in robust anamnestic response across serogroups• Booster dose well-tolerated; no major safety concerns identified | Klein et al.Presented at IDSA 2014, Philadelphia PA;rSBA data on file |
identifier on clinicaltrials.gov
In the MenACWY-CRM antibody persistence studies described in this review, the same hSBA assay was used across all studies, with the same procedures for complement qualification, the same test strains, and with testing performed at a single laboratory. In some studies, the rSBA assay was also used as a supplemental tool. The hSBA and rSBA assays have previously been described in detail.20-23 The hSBA testing was performed by Clinical Laboratory Sciences, GlaxoSmithKline Vaccines GmbH, Marburg, Germany. The rSBA testing was performed at the laboratory of Health Protection Agency, Manchester, UK. Titers were reported as the reciprocal of the lowest dilution that resulted in killing of 50% of test strain bacteria within 60 minutes. The primary measures of immunogenicity were the percentages of subjects who achieved hSBA titers ≥8, and the hSBA geometric mean titers (GMTs), against serogroups A, C, W, and Y reference strains. Although Goldschneider et al. demonstrated that an hSBA titer of 4 was the threshold for clinical protection,24,25 a more conservative threshold of 8 was used for assessment of MenACWY-CRM vaccine-induced immunogenicity in support of vaccine licensure. The same threshold was also used for characterization of bactericidal antibody persistence. The measures of immunogenicity for the additional analyses using rSBA were the percentages of subjects who achieved rSBA titers ≥8, and the rSBA GMTs, against serogroups A, C, W, and Y reference strains. An rSBA titer ≥8 has previously been described as corresponding to a protective threshold following serogroup C vaccination.20,21,23
Persistence of MenACWY-CRM antibodies varies by age and serogroup
Given the rapid onset and progression of meningococcal disease, circulating bactericidal antibodies may be more important for protection against invasive disease than immune memory, since anamnestic antibody responses following meningococcal exposure may not yield protective titers in time to prevent invasive disease. Indeed, investigations of meningococcal serogroup C vaccination failures revealed no deficiency in the magnitude of anamnestic antibody responses, suggesting that vaccination failure may have been due instead to disease progression being more rapid than the immune response.26,27 Therefore, the study of long-term persistence of protective antibody titers becomes crucial to define the potential duration of protection after primary vaccination and to assess the need for and timing of booster doses.
Persistence of antibodies after a single dose of MenACWY-CRM
Four clinical trials conducted in adolescents 11–18 y of age13-15 and children 6–10 and 2–5 y of age12,16 show a trend of high hSBA titers immediately post-vaccination, with an age- and serogroup-specific decline in titers up to 1 year, followed by relatively stable levels up to 5 y post-vaccination. In adolescents and children 2 y of age and older, the waning of bactericidal antibody titers over time was most pronounced for serogroup A, and, to a lesser extent, serogroup C.
At one year after vaccination, antibody titers to serogroup W remained largely unchanged (Fig. 1; lower left pane). Antibody titers to serogroups C and Y waned, with greater decreases seen in the younger age groups (Fig. 1; top and lower right panels). Antibody titers against serogroup A, compared to those against serogroups C, W and Y, declined to a greater degree in all age groups, again with persistence being lowest in the youngest subjects (Fig. 1; top left panel).
Figure 1.
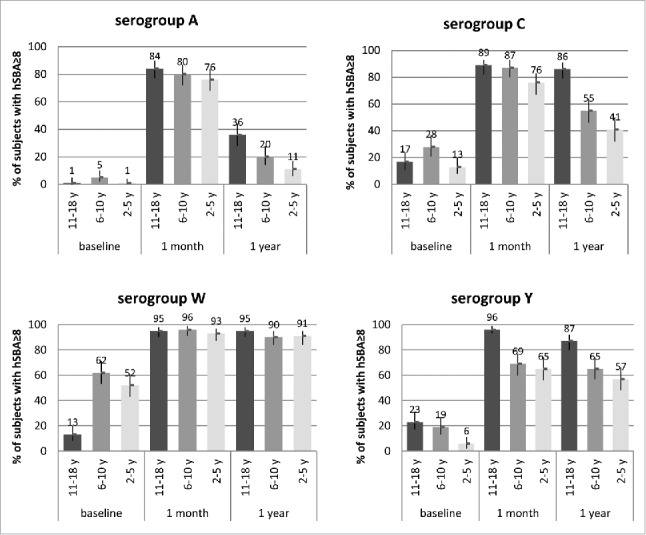
Percentages of subjects with SBA titers ≥8 and 95% CIs (error bars) at baseline (pre-vaccination), and 1 month and 1 y after 1 dose of MenACWY-CRM given to adolescents (11–18 y at time of vaccination; Study 1) and children (2–5 and 6–10 y at time of vaccination; Study 2), by serogroup.
At 5 y after vaccination, titers against serogroups C, W and Y remained fairly stable relative to the one year timepoint, with substantial proportions of adolescents and children retaining hSBA antibody titers ≥8 against these serogroups (Fig. 2). Antibodies against serogroup A were low across age groups (Fig. 2; top left panel).
Figure 2.
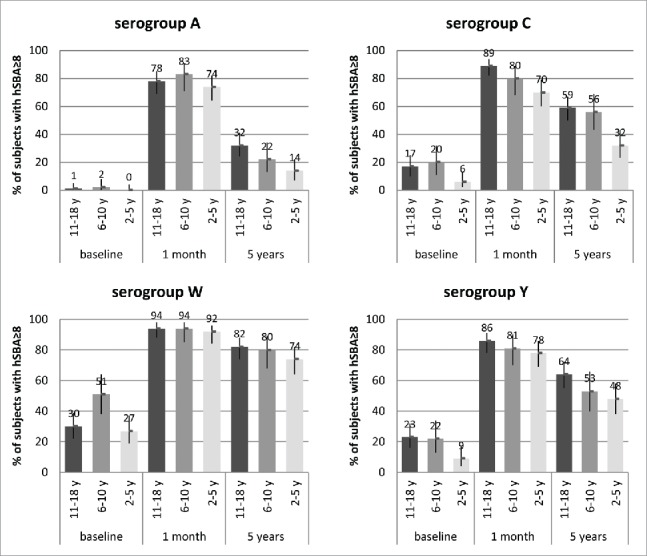
Percentages of subjects with SBA titers ≥8 and 95% CIs (error bars) at baseline (pre-vaccination), 1 month, and 5 y after 1 dose of MenACWY-CRM given to adolescents (11–18 y of age at the time of vaccination; Study 3) and children (2–5 and 6–10 y at the time of vaccination; Study 4), by serogroup.
In Study 3, antibody persistence in adolescents was assessed at 21 months,13 3 years,14 and 5 y after vaccination. After an initial decline by 21 months, substantial proportions of subjects retained hSBA antibody titers ≥8 against serogroups C (≥59%), W (≥82%), and Y (≥64%) at both 3 and 5 years after vaccination. Antibodies to serogroup A declined rapidly, but hSBA titers ≥8 were still present in 32% of subjects at 5 y.
In Study 4,16, at 5 y after initial vaccination, older children (6–10 y of age) demonstrated greater retention of bactericidal antibodies (Fig. 2) against serogroups C and Y compared with the younger cohort (2–5 years). Against serogroup C, 56% of older children and 32% of younger children had hSBA titers ≥8. Similarly, 53% of older children and 48% of younger children had hSBA titers ≥8 against serogroup Y. Antibody persistence against serogroup W was uniformly high across both age cohorts (74–80% of subjects with hSBA titers ≥8), while residual antibody titers against serogroup A were low in both age cohorts (14–22% of children with titers ≥8), although hSBA GMTs against serogroup A were ∼1.5- to 2-fold higher than pre-vaccination levels.
In summary, data from 4 clinical trials shows that waning of serum bactericidal antibody titers after a single dose of MenACWY-CRM is serogroup-specific. Studies conducted using other conjugated quadrivalent meningococcal vaccines have shown similar trends, with serum bactericidal antibody levels to serogroups A, C, W and Y decreasing over time, with serogroup-specific rates of decay (NLM Identifier: NCT01442675).28
Persistence after multi-dose vaccination series in infants and toddlers
In toddlers given a 2-dose primary series of MenACWY-CRM at 6–8 months and 12 months of age, antibody levels declined by 7 months after vaccination and were higher for serogroups C, W and Y (>70% of subjects with titers ≥8) than for serogroup A (31% of subjects with titers ≥8) (Fig. 3) (NLM Identifier: NCT00667602). Geometric mean titers were also modest across serogroups. Similarly, by 6 months after a 3-dose series of MenACWY-CRM given at 2, 4 and 6 months of age in infants,9,10 antibody titers declined to different degrees across serogroups, with more than half of the infants retaining antibody titers ≥8 against serogroups C, W and Y at 6 months after vaccination (Fig. 3). Persistence of antibodies at 6 months was lowest (12% of subjects with titers ≥8) against serogroup A, in spite of a robust immune response 1 month after the third dose.
Figure 3.
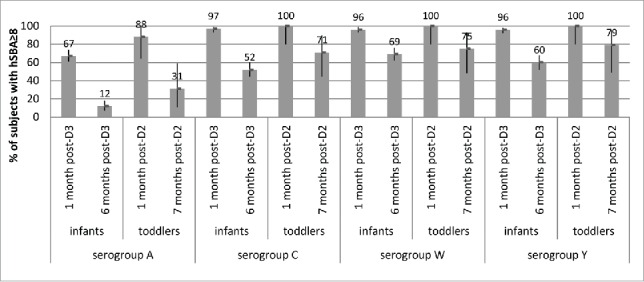
Percentages of subjects with hSBA titers ≥8 and 95% CIs (error bars) at baseline (pre-vaccination) and 1 month and 6 months after 3 doses of MenACWY-CRM given to infants at 2, 4, and 6 months of age (Study 6), and at 1 month and ∼7 months after 2 doses of MenACWY-CRM given to toddlers at 6–8 months and 12 months of age (Study 5), by serogroup.
Long-term persistence of antibodies after vaccination in infancy was also moderate to high across serogroups. In infants given a 4-dose primary vaccination series of MenACWY-CRM, and in toddlers given a 2-dose series, ≥56% of subjects had hSBA≥8 against serogroups W and Y through 5 y of age.18 However, levels of circulating antibodies against serogroup C declined by 5 y of age. Indeed, waning of antibody titers against serogroup C over time is a well-known phenomenon across age groups.30,31 Persistence of antibodies against serogroup A was the lowest across serogroups, consistent with trends seen across studies (Fig. 4).
Figure 4.
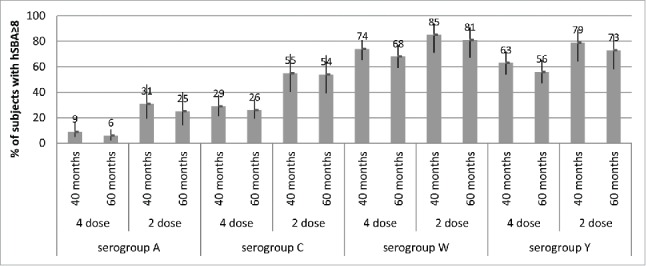
Percentages of subjects with hSBA titers ≥8 and 95% CIs (error bars) at 40 months and 60 months of age (Study 7), after either 4 doses (given at 2, 4, 6 and 12/13 months of age) or 2 doses (given at 12/13 and 15 months of age) of MenACWY-CRM given to infants in Study 3, by serogroup.
Persistence of antibodies in infants may depend on age at last vaccination
It is notable that, in Study 7, children given a 2-dose primary series in the second year of life retained higher levels of bactericidal antibodies 5 y later than those who received 4 vaccinations in the first year of life (Fig. 4).18 For serogroup C, the percentages of subjects with hSBA titers ≥8 at 60 months of age were 54% for those who started the 2-dose series at 12 months of age, and 26% for those who started the 4-dose series at 2 months of age. Against serogroup A, 25–31% for those given 2 toddler doses and 6–9% of those given 4 infant doses had hSBA titers ≥8 over the 40- and 60-month timepoints. These results suggest that age at the time of the last vaccination may exert a greater impact on persistence than the number of priming doses, potentially due to greater maturity of the immune system in toddlers versus infants. This observation is underscored by previous data showing that antibody persistence against serogroup C in older age groups usually exceeds that seen in younger children, toddlers or infants, presumably due to to a greater degree of immunological maturity.32 Indeed, increasing the dosing interval in infants and young children may be more effective in inducing sustained protection than increasing the number of priming doses. However, long-term persistence of antibodies is not the only factor to consider in determining the optimal number and timing of doses administered in infants; the benefit of early protection in the first months of life, when the incidence of IMD is the highest, may dictate the need fora multi-dose infant schedule. Decisions regarding infant and toddler meningococcal vaccination schedules should take into account the need for both immediate and long-term protection, and the prevailing epidemiological conditions.
Rapid waning of serogroup A responses after single or multiple doses of MenACWY-CRM
Our data show a relatively rapid decline of serogroup A hSBA antibody titers after vaccination across age groups. A decline in antibodies against serogroup A has also been seen in clinical trials of other conjugated meningococcal vaccines (NLM Identifier: NCT01442675).28 In fact, in Study 5 (data not included in this review), hSBA antibodies against serogroup A declined to a similar degree over a 5 y period, both in subjects given MenACWY-CRM and those given the meningococcal ACWY-D conjugate vaccine (Menactra®).13-15 This phenomenon has also been seen in toddlers given 1 dose of the meningococcal ACWY-TT vaccine (Nimenrix®), with only 20.6% of healthy toddlers 12 months of age retaining hSBA antibody titers ≥4 against serogroup A at 1 y after vaccination33 and only 21.8% of healthy toddlers 12–23 months of age retaining hSBA antibody titers against serogroup A at 3 y after vaccination.34
Laboratory and assay considerations in assessment of persistence against serogroup A
Antibody decay rates also depend on the serological assay used for measurement of antibody titers. Several types of serological assays have historically been used for assessment of meningococcal antigen-specific immunity, including assays that measure complement-mediated bacterial killing using endogenous and exogenous complement (rabbit or human), anti-capsular antibody levels, or opsonophagocytosis,35 and assays using different animal protection models.36-38
Although the hSBA assay is the most commonly used serologic marker of protection, this assay is difficult to standardize due to the nature of the reagents used.39-41 The bactericidal assay using complement derived from baby rabbit serum (rSBA) is a widely accepted alternative to the hSBA. The rSBA assay generally yields higher titers than are seen in the hSBA. Using the observed effectiveness of the MenC conjugate vaccine in the UK, and comparing seroconversion rates in clinical serum samples collected from vaccinated individuals of all ages, the serological correlate of protection for the rSBA assay has been established as a titer ≥8.20,39 While hSBA antibodies against serogroup A wane rapidly over time, comparable decreases in rSBA titers against serogroup A are not seen. rSBA titers against serogroup A are consistently higher compared to hSBA, and show little decline over time. In adolescents given 1 dose of MenACWY-CRM, rSBA titers ≥8 against serogroup A were seen in all subjects at 1 y after vaccination (data on file). Furthermore, at 21-month13 and 3-year14 timepoints after a single vaccination in adolescents, there were sustained high percentages of subjects (96–99%) with rSBA titers ≥8 against serogroup A, compared with low percentages of subjects with hSBA titers ≥8 (40% at 21 months and 37% at 3 years). This difference in antibody persistence between the hSBA and rSBA assays for serogroup A was not observed for the other serogroups at any timepoint after vaccination.
In children given 4 doses of MenACWY-CRM in the first year of life or 2 doses in the second year of life, antibody persistence at 5 y after the vaccination series was also high when assessed using the rSBA assay,19 with 80–96% of subjects retaining rSBA titers ≥8 across both groups (Fig. 5). As was seen in other age groups, hSBA and rSBA titers were comparable for serogroups C and Y.18,19
Figure 5.
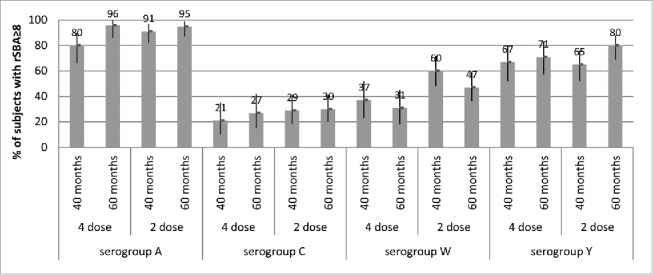
Percentages of subjects with rSBA titers ≥8 and 95% CIs (error bars) at 40 months and 60 months of age (Study 7), after either 4 doses (given at 2, 4, 6 and 12/13 months of age) or 2 doses (given at 12/13 and 15 months of age) of MenACWY-CRM given to infants in Study 3, by serogroup.
Similar findings have previously been described by other investigators.21 Indeed, while both assays have been used variably across clinical studies for meningococcal vaccines, several studies of antibody persistence preferentially utilize the rSBA assay.42,46 A correlation between elevated rSBA titers and protection against serogroup A IMD was also recently suggested by epidemiologic surveillance studies conducted in Africa after a mass-vaccination campaign using the meningococcal serogroup A conjugate vaccine MenAfriVac®. After the vaccination campaign, in the setting of elevated rSBA antibody titers in the population, there were no new cases of IMD, and rates of serogroup A carriage were low.47,48 Another recent study on MenAfriVac® use in the African population has indicated a 94% reduction in the crude incidence rate of meningococcal serogroup A disease with a 98% decrease in the prevalence of MenA carriage.49 Interestingly, antibodies against serogroup A at 1 y after vaccination with MenAfriVac® declined sharply when assessed using hSBA but remained high when assessed using rSBA,50 indicating that hSBA antibody levels in these vaccinated populations may not correlate directly with the observed efficacy of the vaccine and protection against invasive disease. Based on these observations, one might speculate that hSBA may not be the ideal assay for assessing persistence against serogroup A, and that hSBA titers may underestimate actual protection against disease.
Therefore, the selection of an optimal serological assay or, perhaps a battery of assays, remains an important consideration for meningococcal vaccine development. Indeed, different attributes of the immune response may best be evaluated with different assays, adding a level of complexity to the assessments. The mechanism of protection against disease in the absence of hSBA seroprotective antibody titers can be assessed in the whole blood assay, which measures bacterial survival directly in a sample of uncoagulated blood, allowing the measurement of sub-bactericidal concentrations of functional antibodies.51 A technically easier functional assay is opsonophagocytosis (OPA) which uses the hSBA assay platform with the addition of white blood cells.35 Both of these assays are exquisitely sensitive, although technically challenging, and adapting these assays for testing the large number of clinical samples typically generated in a vaccine trial is not feasible. An alternative opsonophagocytic assay platform which could potentially be adopted for higher throughput of clinical samples is a flow cytometry-based assay with the opsonophagocytic activity of the serum antibodies measured as respiratory burst; this assay also using live meningococci as the target cells and human polymorphonuclear neutrophils (PMNs) from donors.52 Another option that may be considered is the use of fixed fluorescently labeled bacteria with the donor PMN's.53 Note that unlike the serum bactericidal assay, there are no formal guidelines for standardization of meningococcal opsonophagocytic assays nor correlate of protection.
Response to a booster dose of MenACWY-CRM
In 3 separate clinical trials conducted across age groups,13-16,18,19 a single booster dose of MenACWY-CRM administered 3 to 5 y after primary vaccination induced a robust anamnestic response in all age groups.
In adolescents, a booster dose of MenACWY-CRM, given 3 y after primary vaccination at 11–18 years,14 induced bactericidal antibody titers ≥8 in all subjects against all serogroups (Fig. 6), while hSBA GMTs were 18- to 121-fold higher than pre-booster titers, with the largest gain in titers seen for serogroup A. Notably, a robust booster response is not specific to the priming vaccine; administration of a booster dose of MenACWY-CRM generated an equally robust immune response in adolescents primed with either MenACWY-CRM or Menactra®.14
Figure 6.
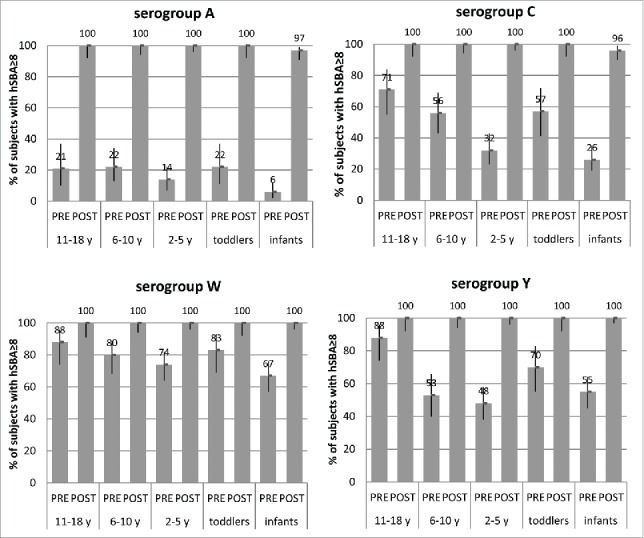
Percentages of subjects with hSBA titers ≥8 and 95% CIs (error bars) pre-booster and at 1 month after booster dose of MenACWY-CRM given 3 y after a single primary dose of MenACWY-CRM in adolescents aged 11–18 y (Study 3) or 5 y after a single dose in children aged 2–5 and 6–10 y of age (Study 4), or 5 y after 2-dose primary vaccination series in toddlers 12–24 months of age or 4-dose primary series in infants aged 2 months (Study 7), by serogroup.
Long-term persistence of the immune response to a booster dose was also extremely robust in adolescents, with minimal decline in antibody titers to serogroups C, W and Y (95–100% of subjects with hSBA titers ≥8) 2 y after the booster dose, and a modest decrease (71% of subjects with hSBA titers ≥8) against serogroup A15. For each serogroup, bactericidal antibody titers 2 y after a booster dose15 were higher than those seen ∼2 y after the primary dose.13
When adolescents were administered a MenACWY-CRM booster 5 y after primary vaccination,54 nearly all subjects (98%-100%) had hSBA titers ≥8 as early as 7 d after the booster dose. In comparison, 64–90% of age-matched naïve subjects had antibody titers ≥8 at 7 d after a single priming dose.54
A booster dose of MenACWY-CRM given 5 y after a single-dose initial vaccination in children 2–5 and 6–10 y of age16 also induced hSBA titers ≥8 in all subjects against all 4 serogroups (Fig. 6). Similarly, in Study 718, a booster dose given at 5 y of age after 2 toddler doses of MenACWY-CRM induced hSBA titers ≥8 in all subjects (Fig. 6). Among infants vaccinated with 4 doses of MenACWY,18 all had post-booster titers ≥8 against serogroups W and Y, and 96–97% had titers ≥8 against serogroups A and C (Fig. 6). Although one cannot underestimate the importance of circulating antibodies for the prevention of IMD, these data support the argument that immunological memory may also play an important role in protection against meningococcal disease.55
A booster dose of quadrivalent meningococcal vaccine is currently recommended for adolescents and young adults, following primary MenACWY vaccination in the United States,3 and following the childhood MenC vaccination in the UK.4 Based on the pattern of antibody waning demonstrated across the studies included in this manuscript, it is possible to conclude that a benefit from booster vaccination may be expected in other age groups as well. Given the varying vaccination schedules and recommendations for quadrivalent meningococcal vaccinations around the world, the need for and timing of a booster dose should be based on national recommendations for primary vaccination and prevailing epidemiologic conditions.
Safety after a booster dose of MenACWY-CRM
Safety after vaccination was also assessed in all of the studies included in this review. Long-term safety assessments did not indicate any specific safety concern, across studies and age groups.9,10,13-16,18 MenACWY-CRM was also well-tolerated as a booster, with no difference in local or systemic reactogenicity compared to single- or multiple-dose primary vaccinations.14,16,18 In the studies involving a booster vaccination (studies 3, 4 and 7), the most commonly reported local reaction after booster was pain at the injection site (or tenderness in infants), while the most commonly reported systemic reaction was irritability in infants, toddlers and young children, and headache in older children and adolescents. There was no appreciable difference in the pattern or frequency of post-injection reactions among subjects receiving a booster injection and those receiving a primary vaccination (age-matched control subjects). Unsolicited AEs after a booster vaccination were reported at rates similar to those seen after primary vaccinations with MenACWY-CRM.
Limitations
The data included in this review are, for the most part, fairly comparable across trials; however there are several limitations that may influence the interpretation of these data. First, the majority of subjects in the studies included in this review were enrolled in the United States. This allowed for more meaningful comparisons of persistence between studies and age groups, but, as a result, these data may not entirely reflect trends in antibody persistence in other regions, populations and epidemiologic settings. Second, although the hSBA and rSBA assays used in these studies were performed in the same laboratories with the same laboratory procedures, a certain degree of caution should be exercised while making inter-study comparisons, especially given that these results were generated over several years.
Finally, in the work by Goldschneider et al.,24,25 a titer of 4 with intrinsic human complement correlated with and was established as the threshold of clinical protection, while a more conservative threshold of 8 was selected as a clinical endpoint for licensure of MenACWY conjugate vaccines. Our data, and those generated for other licensed meningococcal vaccines, demonstrate that the increase of the threshold has had very limited impact on the assessment of immunogenicity at 1 month after primary vaccination. However, the conservative threshold of 8 was not selected or optimized for assessment of antibody persistence, and may therefore be less appropriate for this purpose.
Conclusion
In summary, primary vaccination with MenACWY-CRM vaccine induces a robust immune response across age groups, which is maintained to a considerable degree for up to 5 y. Antibody persistence is greatest in older children and adolescents. While antibody titers against serogroup A decline over time as measured by the hSBA assay, titers measured by the rSBA assay suggest that protection against this serogroup may be sustained in all age groups. All primary vaccination schedules induced immunologic priming, as demonstrated by anamnestic responses to revaccination at 3 to 5 y after primary vaccination.
Abbreviations
- ACP
Advisory Committee on Immunization Practices
- ECs
Ethics Committees
- GMTs
Geometric Mean Titers
- hSBA
human Serum Bactericidal Assay
- IMD
Invasive Meningococcal Disease
- IRBs
Institutional Review Boards
- MenACWY
Quadrivalent Conjugate Meningococcal Vaccine
- MenACWY-CRM
Quadrivalent Meningococcal CRM197-conjugated Vaccine
- OPA
Opsonophagocytosis Assay
- rSBA
rabbit Serum Bactericidal Assay
Disclosure of potential conflicts of interest
Roger Baxter (or his associated institute) received research grants for the conduct of the study, but has no other financial interests. At the time of the study Pavitra Keshavan, Jo Anne Welsch, Linda Han and Igor Smolenov were full-time employees of Novartis group companies (now GSK group companies).
Acknowledgment
The authors would like to thank Ellen Ypma and Elvira Ponce (both employees of GSK Vaccines) for review of the manuscript and for providing valuable suggestions, and Nga Tong for manuscript coordination. Editorial assistance was provided by Philip Matthews, PhD of Zoetic Science, an Ashfield company.
References
- [1].Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27 Suppl 2:B51-863; PMID:19477562; http://dx.doi.org/ 10.1016/j.vaccine.2009.04.063 [DOI] [PubMed] [Google Scholar]
- [2].Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:853-61; PMID:21075057; http://dx.doi.org/ 10.1016/S1473-3099(10)70251-6 [DOI] [PubMed] [Google Scholar]
- [3].Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE, Centers for Disease Control and Prevention (CDC) . Prevention and control of meningococcal disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Recommendations Rep March 2013. 66(RR02);1-22 [accessed November2015]. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6202a1.htm [PubMed] [Google Scholar]
- [4].National Health Service, UK The NHS vaccination schedule, April 2014. [accessed November2015]. Available at: http://www.nhs.uk/Conditions/vaccinations/Pages/vaccination-schedule-age-checklist.aspx [Google Scholar]
- [5].Departamento de Actualización Profesional Boletín Official 33.085, 12 March 2015. [accessed November2015] Available at http://www.colfarsfe.org.ar/newsfiles/marzo2015/Disposiciones12-03-15.pdf [Google Scholar]
- [6].Ministry of Health Portal, Kingdom of Saudi Arabia Basic vaccination schedule [accessed November2015]. Available at: http://www.moh.gov.sa/en/HealthAwareness/EducationalContent/HealthTips/Pages/Tips-005.aspx [Google Scholar]
- [7].Reisinger KS, Baxter R, Block SL, Shah J, Bedell L, Dull PM. Quadrivalent meningococcal vaccination of adults: phase III comparison of an investigational conjugate vaccine, MenACWY-CRM, with the licensed vaccine, Menactra. Clin Vaccine Immunol 2009; 16:1810-15; PMID:19812260; http://dx.doi.org/ 10.1128/CVI.00207-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Halperin SA, Gupta A, Jeanfreau R, Klein NP, Reisinger K, Walter E, Bedell L, Gill C, Dull PM. Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age. Vaccine 2010; 28:7865-72; PMID:20943209; http://dx.doi.org/ 10.1016/j.vaccine.2010.09.092 [DOI] [PubMed] [Google Scholar]
- [9].Klein NP, Reisinger KS, Johnston W, Odrljin T, Gill CJ, Bedell L, Dull P. Safety and immunogenicity of a novel quadrivalent meningococcal CRM-conjugate vaccine given concomitantly with routine vaccinations in infants. Pediatr Infect Dis J 2012; 31:64-71; PMID:22094635; http://dx.doi.org/ 10.1097/INF.0b013e31823dce5c [DOI] [PubMed] [Google Scholar]
- [10].Tregnaghi M, Lopez P, Stamboulian D, Graña G, Odrljin T, Bedell L, Dull PM. Immunogenicity and safety of a quadrivalent meningococcal polysaccharide CRM conjugate vaccine in infants and toddlers. Int J Infect Dis 2014; 26:22-e30; PMID:24980467; http://dx.doi.org/ 10.1016/j.ijid.2014.03.1390 [DOI] [PubMed] [Google Scholar]
- [11].Johnston W, Essink B, Kirstein J, Forleo-Neto E, Percell S, Han L, Keshavan P, Smolenov I. Comparative Assessment of a 2-dose and a 1–dose vaccination series of a Quadrivalent Meningococcal conjugate vaccine (MenACWY-CRM) in children 2-10 years of age. Pediatr Infect Dis J 2016;35:e19-27; PMID:26398741; http://dx.doi.org/21339701 10.1097/INF.0000000000000931 [DOI] [PubMed] [Google Scholar]
- [12].Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin 2010; 6:881-7; PMID:21339701; http://dx.doi.org/ 10.4161/hv.6.11.12849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baxter R, Reisinger K, Block SL, Izu A, Odrljin Dull P. Antibody persistence and booster response of a quadrivalent meningococcal conjugate vaccine in adolescents. J Pediatr 2014; 164:1409-15; PMID:24657122; http://dx.doi.org/ 10.1016/j.jpeds.2014.02.025 [DOI] [PubMed] [Google Scholar]
- [14].Baxter R, Reisinger K, Block S, Percell S, Odrljin T, Dull PM, Smolenov I. Antibody persistence after primary and booster doses of a quadrivalent meningococcal conjugate vaccine in adolescents. Pediatr Infect Dis J 2014; 33:1169-76; PMID:24911896; http://dx.doi.org/ 10.1097/INF.0000000000000438 [DOI] [PubMed] [Google Scholar]
- [15].Block SL, Christensen S, Verma B, Xie F, Keshavan P, Dull PM, Smolenov I. Antibody persistence 5 years after vaccination at 2 to 10 years of age with Quadrivalent MenACWY-CRM conjugate vaccine, and responses to a booster vaccination. Vaccine 2015:33:2175-82; PMID:25744224; http://dx.doi.org/ 10.1016/j.vaccine.2015.02.049 [DOI] [PubMed] [Google Scholar]
- [16].Klein NP, Block SL, Johnston W, Percell S, Han L, Dull PM, et al.. Persistence of meningococcal bactericidal antibodies and booster response at 60-months of age in children who received infant or toddler doses of MenACWY-CRM conjugate vaccine. Presented at the Infectious Diseases Society of America 2014, Philadelphia PA, USA. [Accessed February 9, 2016.] Available at: https://idsa.confex.com/idsa/2014/webprogram/Paper45999.html. [Google Scholar]
- [17].Klein NP, Block SL, Johnston W, Percell S, Keshavan P, Dull PM et al.. Persistence of meningococcal antibodies after infant and toddler MenACWY-CRM conjugate vaccine assessed with serum bactericidal assays using human and rabbit complement Presented at Meningitis Res Found 2015, London UK. [Accessed February 9, 2016] Available at: http://www.meningitis.org/assets/x/57410 [Google Scholar]
- [18].Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for the use of meningococcal serogroup C conjugate vaccines in the United Kingdom: Reevaluation of the correlates of protection. Infect Immun 2001; 59:1568-73; http://dx.doi.org/ 10.1128/IAI.69.3.1568-1573.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Findlow H, Plikaytis BD, Aase A, Bash MC, Chadha H, Elie C, Laher G, Martinez J, Herstad T, Newton E, et al.. Investigation of different group A immunoassays following one dose of meningococcal group A conjugate vaccine or A/C polysaccharide vaccine in adults. Clin Vaccine Immunol 2009; 16:969-77; PMID:19474264; http://dx.doi.org/ 10.1128/CVI.00068-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McIntosh ED, Bröker M, Wassil J, Welsch JA, Borrow R. Serum bactericidal antibody assays - The role of complement in infection and immunity. Vaccine 2015; 33:4414-21; PMID:26187262; http://dx.doi.org/ 10.1016/j.vaccine.2015.07.019 [DOI] [PubMed] [Google Scholar]
- [21].Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from post licensure surveillance in England. Clin Diagn Lab Immunol 2003; 10:780-6; PMID:12965904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the Meningococcus I. The role of humoral antibodies. J Exp Med 1969; 129:1307-26; PMID:4977280; http://dx.doi.org/ 10.1084/jem.129.6.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the Meningococcus II. Development of natural immunity. J Exp Med 1969; 129:1327-48; PMID:4977281; http://dx.doi.org/ 10.1084/jem.129.6.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Auckland C, Gray S, Borrow R, Andrews N, Goldblatt D, Ramsay M, Miller E. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J Infect Dis 2006; 194:1745-52; PMID:17109348; http://dx.doi.org/ 10.1086/509619 [DOI] [PubMed] [Google Scholar]
- [25].Snape MD, Kelly DF, Salt P, Green S, Snowden C, Diggle L, Borkowski A, Yu LM, Moxon ER, Pollard AJ. Serogroup C meningococcal glycoconjugate vaccine in adolescents: persistence of bactericidal antibodies and kinetics of the immune response to a booster vaccine more than 3 years after immunization. Clin Infect Dis 2006; 43:1387-94; PMID:17083009; http://dx.doi.org/ 10.1086/508776 [DOI] [PubMed] [Google Scholar]
- [26].Baxter R, Baine Y, Kolhe D, Baccarini CI, Miller JM, Van der Wielen M. Five-year antibody persistence and booster response to a single dose of meningococcal A, C, W, and Y tetanus toxoid conjugate vaccine in adolescents and young adults: an open, randomized trial. Pediatr Infect Dis J 2015; 34:1236-43; PMID:26237742; http://dx.doi.org/ 10.1097/INF.0000000000000866 [DOI] [PubMed] [Google Scholar]
- [27].De Wals P. Immunization strategies for the control of serogroup C meningococcal disease in developed countries. Expert Rev Vaccines 2006; 5:269-75; PMID:16608426; http://dx.doi.org/ 10.1586/14760584.5.2.269 [DOI] [PubMed] [Google Scholar]
- [28].Borrow R, Abad R, Trotter C, van der Klis FR, Vazquez JA. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine 2013; 31:4477-86; PMID:23933336; http://dx.doi.org/ 10.1016/j.vaccine.2013.07.083 [DOI] [PubMed] [Google Scholar]
- [29].Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, Langley JM, McNeil S, Dull PM, Ceddia F, et al.. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA 2008; 299:173-84; PMID:18182599; http://dx.doi.org/ 10.1001/jama.2007.29-c [DOI] [PubMed] [Google Scholar]
- [30].Klein NP, Baine Y, Bianco V, Lestrate PR, Naz A, Blatter M, Friedland LR, Miller JM. One or two doses of quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine is immunogenic in 9- to 12-month-old children. Pediatr Infect Dis J 2013; 32:760-7; PMID:23348814; http://dx.doi.org/ 10.1097/INF.0b013e31828693c5 [DOI] [PubMed] [Google Scholar]
- [31].Vesikari T, Forsten A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum Vaccines Immunother 2012; 8:1892-903; http://dx.doi.org/ 10.4161/hv.22166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Plested JS, Granoff DM. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin Vaccine Immunol 2008; 15:799-804; PMID:18353918; http://dx.doi.org/ 10.1128/CVI.00036-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Welsch JA, Granoff DM. Naturally acquired passive protective activity against Neisseria meningitidis Group C in the absence of serum bactericidal activity. Infect Immun 2004; 72:5903-09; PMID: 15385492; http://dx.doi.org/ 10.1128/IAI.72.10.5903-5909.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pajon R, Buckwalter CM, Johswich KO, Gray-Owen SD, Granoff DM. A native outer membrane vesicle vaccine confers protection against meningococcal colonization in human CEACAM1 transgenic mice. Vaccine 2015; 33:1317-23; PMID:25662856; http://dx.doi.org/ 10.1016/j.vaccine.2015.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gorringe AR, Reddin KM, Funnell SG, Johansson L, Rytkonen A, Jonsson AB. Experimental disease models for the assessment of meningococcal vaccines. Vaccine 2005; 23:17-18 [DOI] [PubMed] [Google Scholar]
- [36].Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection-serum bactericidal antibody activity. Vaccine 2005; 23:2222-27; PMID:15755600; http://dx.doi.org/ 10.1016/j.vaccine.2005.01.051 [DOI] [PubMed] [Google Scholar]
- [37].Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, Arhin FF, Devi SJ, Frasch CE, Huang JC, et al.. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The ultilaboratory Study Group. Clin Diagn Lab Immunol 1997; 4:156-167; PMID:9067649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Balmer P, Borrow R. Issues surrounding standardization of meningococcal serogroup W135 serology. Vaccine 2007; 25 (Suppl 1):A63-A68; PMID:17544550; http://dx.doi.org/ 10.1016/j.vaccine.2007.04.043 [DOI] [PubMed] [Google Scholar]
- [39].Borja-Tabora C, Montalban C, Memish ZA, Van der Wielen M, Bianco V, Boutriau D, Miller J. Immune response, antibody persistence, and safety of a single dose of the quadrivalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine in adolescents and adults: results of an open, randomised, controlled study. BMC Infect Dis 2013; 13:116; PMID:23510357; http://dx.doi.org/ 10.1186/1471-2334-13-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Østergaard L, Van der Wielen M, Bianco V, Miller JM. Persistence of antibodies for 42 months following vaccination of adolescents with a meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine (MenACWY-TT). Int J Infect Dis 2013; 17: e173-6; http://dx.doi.org/ 10.1016/j.ijid.2012.10.001 [DOI] [PubMed] [Google Scholar]
- [41].Pichichero M, Casey J, Blatter M, Rothstein E, Ryall R, Bybel M, Gilmet G, Papa T. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two- to ten-year-old children. Pediatr Infect Dis J 2005; 24:57-62; PMID:15665711; http://dx.doi.org/ 10.1097/01.inf.0000148928.10057.86 [DOI] [PubMed] [Google Scholar]
- [42].Pichichero M, Papa T, Blatter M, Mitchell D, Kratz R, Sneed J, Bassily E, Casey J, Gilmet G. Immune memory in children previously vaccinated with an experimental quadrivalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J 2006; 25:995-1000; PMID:17072120; http://dx.doi.org/ 10.1097/01.inf.0000243215.46312.4a [DOI] [PubMed] [Google Scholar]
- [43].Vesikari T, Forsten A, Bianco V, Van Der Wielen M, Miller JM. Antibody persistence up to five years after vaccination of toddlers and children between 12 months and 10 years of age with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine. Hum Vaccin Immunother. 2015 Dec;34:e298-307; PMID:26780033; http://dx.doi.org/25472422 10.1097/INF.0000000000000897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yaro S, Tall H, Kpoda H, Ouangraoua S, Trotter C, Njanpop Lafourcade B-M, et al.. Meningococcal seroepidemiology in Burkina Faso, one year after the MenAfriVac® campaign. Presented at the Meningitis Research Foundation Conference 2013, London UK. [Accessed February 9, 2016] Available at: http://www.meningitis.org/assets/x/55419
- [45].Kristiansen PA, Ba AK, Ouédraogo AS, Sanou I, Ouédraogo R, Sangaré L, Diomandé F, Kandolo D, Saga IM, Misegades L, et al.. Persistent low carriage of serogroup A Neisseria meningitidis two years after mass vaccination with the meningococcal conjugate vaccine, MenAfriVac. BMC Infect Dis 2014; 14:663-74; PMID:25472422; http://dx.doi.org/ 10.1186/s12879-014-0663-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Daugla DM, Gami JP, Gamougam K, Naibei N, Mbainadji L, Narbé M, Toralta J, Kodbesse B, Ngadoua C, Coldiron ME, et al.. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet 2014; 383:40-47; PMID:24035220; http://dx.doi.org/ 10.1016/S0140-6736(13)61612-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Price G, Plikaytis B, Hollander A, Mocca B, Carlone G, Findlow H, et al.. Comparison of different serogroup A immunoassays following a single dose of either MenAfriVac or quadrivalent polysaccharide vaccine in healthy Africans 2- to 29- years of age. Presented at International Pathogenic Neisseria Conferences 2014, Asheville NC, USA. [Accessed February 9, 2016] Available at: http://neisseria.org/ipnc/2014/IPNC_2014_abstracts.pdf [Google Scholar]
- [48].Plested JS, Welsch JA, Granoff DM. Ex-vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity. Clin Vaccine Immunol 2009; 16:785-91; PMID:19339487; http://dx.doi.org/ 10.1128/CVI.00007-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Norheim G, Aase A, Caugant DA, Hølby EA, Fritzsønn E, Tangen T, Kristiansen P, Heggelund U, Rosenqvist E. Development and characterization of outer membrane vesicle vaccines against serogroup A Neisseria meningitidis. Vaccine 2005. 23:3762-74; PMID:15893613; http://dx.doi.org/ 10.1016/j.vaccine.2005.02.021 [DOI] [PubMed] [Google Scholar]
- [50].Plested JS, Ferry BL, Coull PA, Makepeace K, Lehmann AK, MacKinnon FG, Griffiths HG, Herbert MA, Richards JC, Moxon ER. Functional opsonic activity of human serum antibodies to inner core lipopolysaccharide (galE) of serogroup B meningococci measured by flow cytometry. Infect Immun 2001. 69:3203-13; PMID:11292742; http://dx.doi.org/ 10.1128/IAI.69.5.3203-3213.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jacobson RM, Jackson LA, Reisinger K, Izu A, Odrljin T, Dull PM. Antibody persistence and response to a booster dose of a quadrivalent conjugate vaccine for meningococcal disease in adolescents. Pediatr Infect Dis J 2013; 32: e170-7; PMID:23114372; http://dx.doi.org/ 10.1097/INF.0b013e318279ac38 [DOI] [PubMed] [Google Scholar]
- [52].Borrow R, Goldblatt D, Finn A, Southern J, Ashton L, Andrews N, Lal G, Riley C, Rahim R, Cartwright K, et al.. Immunogenicity of, and immunologic memory to, a reduced primary schedule of meningococcal C-tetanus toxoid conjugate vaccine in infants in the United Kingdom. Infect Immun 2003; 71:5549-55; PMID:14500473; http://dx.doi.org/ 10.1128/IAI.71.10.5549-5555.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]


