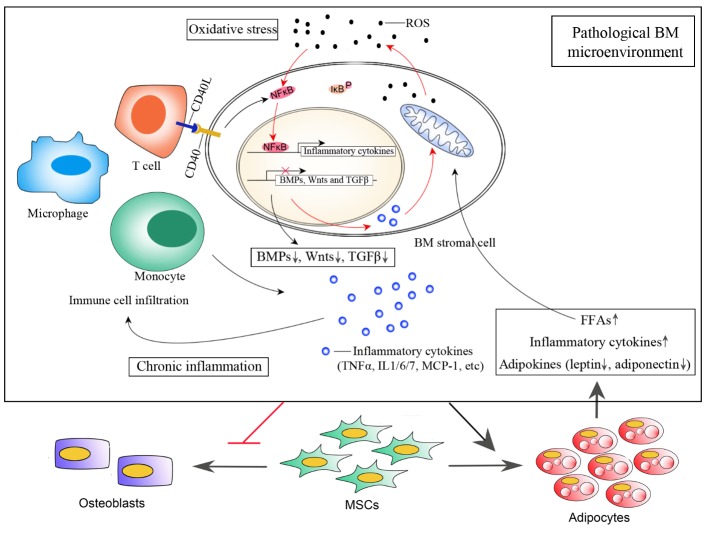
Figure 1.
BM microenvironment under pathological conditions. Aging-associated increase in ROS promotes NF-κB releasing from IκB and subsequent translocation into nucleus where it binds to responsive element to activate transcription of inflammatory cytokines such as TNFα, Il-6 and MCP-1. Elevated level of these inflammatory cytokines in BM results in immune cell infiltration from blood, such as T cells, monocytes and macrophages. CD40/CD40L mediated Cell-cell communication between T cells and BM stromal cells further enhances NF-κB signal, promoting stromal cells express more inflammatory cytokines. Additional inflammatory cytokines such as TNFα and IL-1 secreted from stromal cells as well as infiltrated immune cells also stimulates ROS generation through mitochondrial and NADPH oxidase system, forming a positive feedback loop that contributes to BM oxidative stress and chronic inflammation. These pathological environmental signals shift MSC fate to favor adipocytes over osteoblasts. Accumulated fat further deteriorate BM microenvironment through secreted FFAs, inflammatory cytokines and altered adipokine secretome. Besides, excessive FFAs generate more ROS while oxidation, initiating a vicious cycle that accelerates BM microenvironment deterioration.