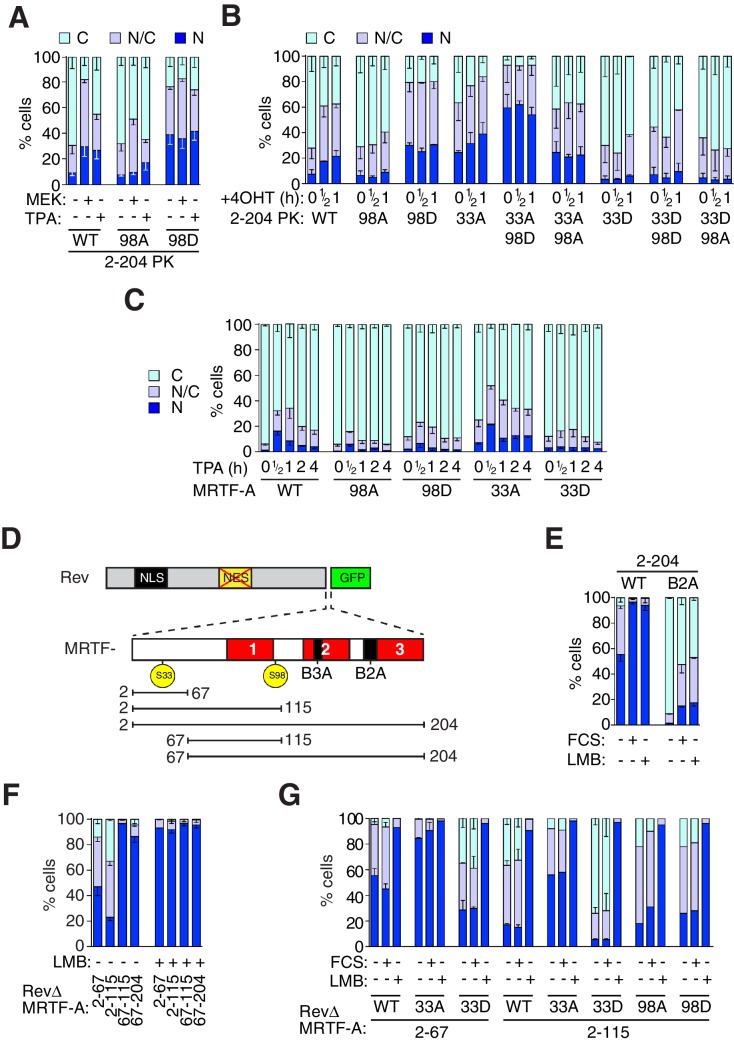
Figure 5. Phosphorylation of S98 and S33 has opposing effects on MRTF-A nuclear accumulation.
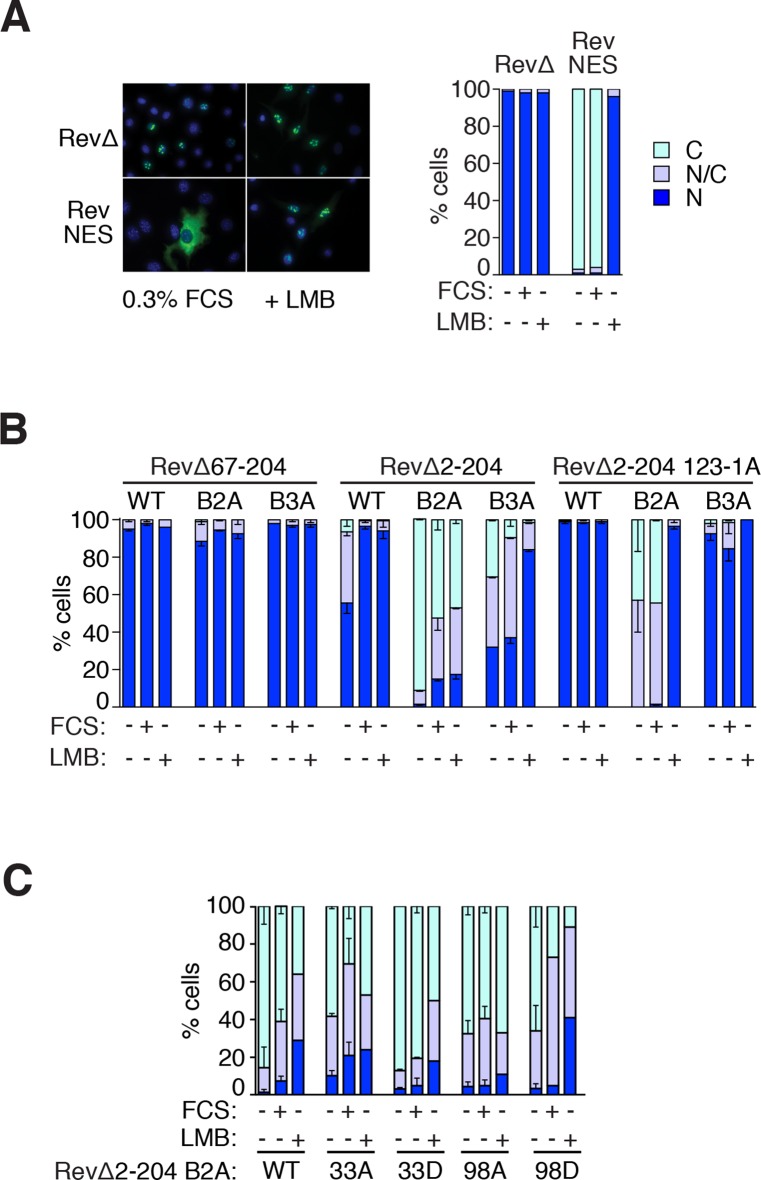
(A,B) Cells expressing MRTF-A derivatives, with or without activated MEK or RafER, were treated as indicated, and their subcellular localisation scored by immunofluorescence. (A) ERK-mediated S98 phosphorylation controls MRTF-A(2–204)PK localisation. Data are mean ± SEM, n = 3. Mutant S98D baseline higher than wildtype or S98A (p<0.01); MEK and TPA potentiate wildtype (p<0.001); 98A increased by MEK (p<0.01; possibly reflecting chronic ERK stimulation); others not significant. Two-way ANOVA with Bonferroni post-test. (B) Alanine substitutions or 'phosphomimetic' aspartate substitutions at S33 and S98 have opposing effects on MRTF-A(2–204)PK subcellular localisation. Data are mean ± half-range, n = 2. Mutant S98D baseline higher than wildtype or S98A (p<0.01); S98D and S33A cooperate to elevate baseline (p<0.001); 4OHT significantly induces wildtype and S33A (both p<0.05); two-way ANOVA with Bonferroni post-test. (C) N-terminal phosphosite mutations affect nucleocytoplasmic shuttling of intact MRTF-A. Data are mean ± half-range, n = 2. TPA treatment significantly increases nuclear localisation of wild-type MRTF-A (p<0.001), 33A (p<0.001) and 98D (p<0.05); one-way ANOVA, Bonferroni multiple comparison test. (D) Schematic representation of the MRTF-A / RevΔGFP derivatives. (E–G) analysis of MRTF-A NES activity by RevΔGFP assay. (E) MRTF-A(2–204) contains a Crm1-dependent NES. Data are mean ± half-range, n = 2. (F) MRTF-A residues 2–67, but not the RPEL domain, function as an NES. Data are mean ± SEM, n = 3. The 2–67 and 2–115 derivatives differ significantly from each other and from 67–115 and 67–204 in resting cells (all p<0.001 except 2–67 vs 67–204, p<0.01), but not following LMB treatment; two-way ANOVA with Bonferroni post-test. (G) Alanine or 'phosphomimetic' aspartate substitutions at S33, but not S98 affect MRTF-A N-terminal NES activity. Data are mean ± half-range, n = 2; absence of error bar shows a single datapoint (ie mean of two technical replicates).