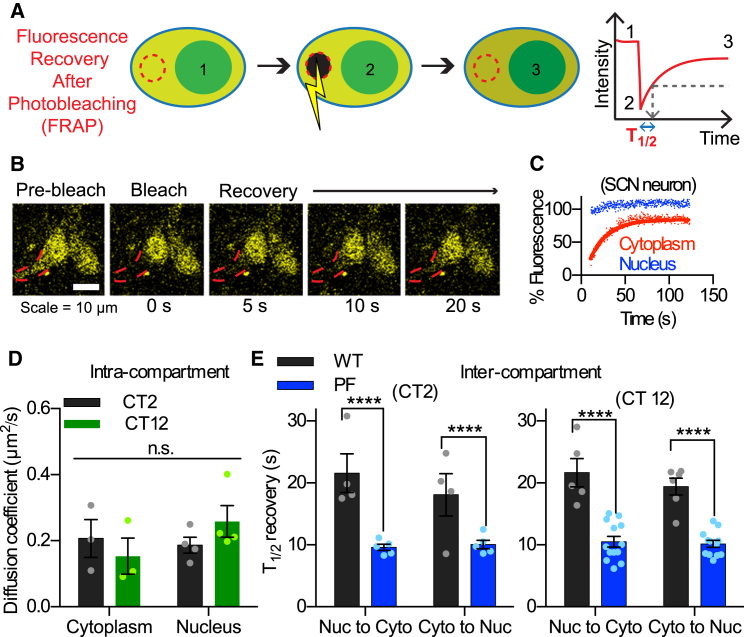
Figure 4.
FRAP Reveals Role of CK1 in Regulating PER2 Mobility in SCN Neurons
(A) A schematic diagram illustrating FRAP as follows: (1) select region and measure pre-bleach fluorescence, (2) photobleach region, and (3) monitor the recovery of fluorescence. The T1/2 is calculated from the recovery curve.
(B) Snapshots from a FRAP experiment show cytoplasmic photobleaching and fluorescence recovery in neurons in an SCN slice.
(C) Fluorescence recovery for a bleached cytoplasmic region (red) and unbleached nuclear region (blue). The latter shows no change in fluorescence over the time course.
(D) FRAP-derived diffusion coefficients (mean ± SEM) within SCN nucleus and cytoplasm were comparable at both peak and trough (CT2 and CT12) of the cycle (nCT2,cyto = 3 slices, nCT12,cyto = 4, nCT2,nuc = 3, and nCT12,nuc = 4; two-way ANOVA). Diffusion coefficients were estimated from FRAP-derived T1/2 measures.
(E) FRAP-derived T1/2 (mean ± SEM) calculated from total cytoplasm (Nuc to Cyto) and total nucleus (Cyto to Nuc) FRAP of SCN neurons at CT2 (left) and CT12 (right). Treatment with PF670462 (1 μM) significantly decreased T1/2 times for PER2::VENUS (nCT2,WT,cyto = 4 slices, nCT2,WT,nuc = 4, nCT2,PF,cyto = 5, nCT2,PF,nuc = 5, nCT12,WT,cyto = 5, nCT12,WT,nuc = 6, nCT12,PF,cyto = 13, and nCT12,PF,nuc = 12; two-way ANOVA with Tukey’s comparison, ∗∗∗∗p < 0.0001).