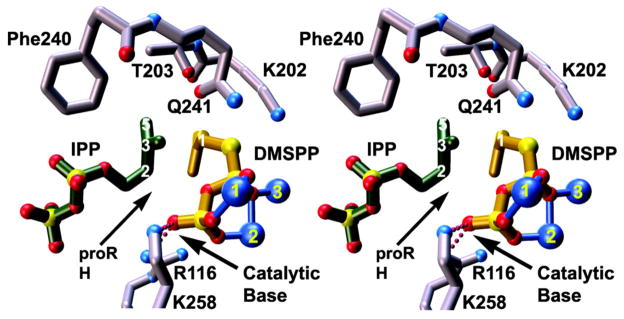
Figure 3.
The active site of E. coli farnesyl diphosphate synthase complexed with three Mg2+ ions (blue spheres 1, 2, and 3), dimethylallyl-S-thiolodiphosphate (DMSPP, yellow), and isopentenyl diphosphate (IPP, green). Metal coordination and hydrogen bond interactions are indicated by solid blue and dotted magenta lines, respectively. The diphosphate group of DMSPP is oriented for abstraction of the pro-R hydrogen from IPP following the chain elongation reaction. Reprinted with permission from: Hosfield, D. J., Zhang, Y., Dougan, D. R., Broun, A., Tari, L. W., Swanson, R. V. & Finn, J. Structural basis for bisphosphonate-mediated inhibition of isoprenoid biosynthesis. J. Biol. Chem. 279, 8526–8529 (2004). Copyright 2004 The American Society for Biochemistry & Molecular Biology.