Figure 8.
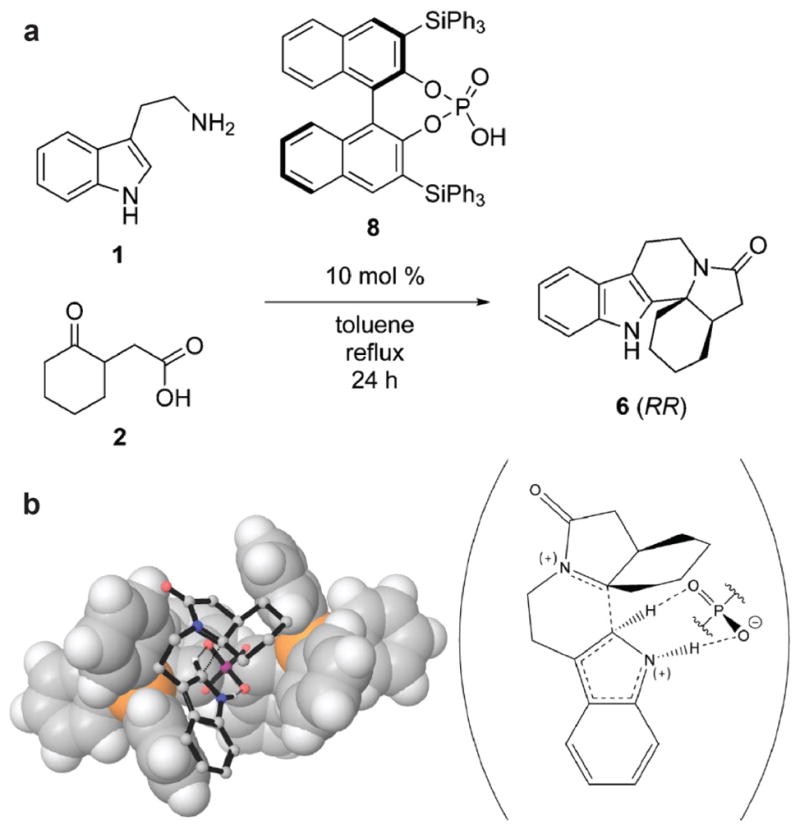
(a) Pictet-Spengler reaction of tryptamine 1 and (2-oxocyclohexyl)acetic acid 2 catalyzed by BINOL derivative 3,3′-bis-(triphenylsilyl)-1,1′-bi-2-naphthol phosphoric acid 8. (b) Calculated transition state structure for the Pictet-Spengler reaction of 1 and 2 (stick figures) catalyzed by 8 (van der Waals surface). The molecular scheme on the right illustrates the proton abstraction by phosphate that quenches the carbocation intermediate. Reprinted with permission from: Overvoorde, L. M., Grayson, M. N., Luo, Y., & Goodman, J. M. (2015) Mechanistic insights into a BINOL-derived phosphoric acid-catalyzed asymmetric Pictet-Spengler reaction. J. Org. Chem. 80, 2634–2640 (2015). Copyright 2015 American Chemical Society.
