Abstract
Objective
Assess cross-sectional relationships between BMI, waist circumference (WC), pericardial (PAT), visceral (VAT), and subcutaneous adipose tissue (SAT) volumes with calcified plaque (CP) in African Americans (AAs) and European Americans (EAs) with type 2 diabetes.
Methods
Computed tomography measured PAT, VAT, SAT, and CP in coronary arteries (CAC), carotid arteries, and aorta. Generalized estimating equations models were fitted to test for associations between adiposity and CP, stratified by ethnicity while accounting for familial correlations.
Results
AAs (N=753) vs. EAs (N=562) had significantly lower PAT and VAT, despite equal or higher BMI. In multivariable models adjusting for age, gender, education, HbA1c, statins, smoking, CVD, hypertension, nephropathy, and CRP, PAT positively associated with presence of CAC in AAs (p<0.001), not EAs (p=0.68; ethnicity-interaction p<0.01). Inverse associations were detected between SAT and severity of aorta CP (p<0.01) in AAs, and between BMI, WC, and SAT with severity of aorta CP in all participants.
Conclusions
Ethnic- and gender-specific differences in BMI, WC, PAT, SAT, and VAT were present in AAs and EAs with diabetes. Only PAT was positively associated with CAC in AAs, paradoxical inverse associations were seen between several other adiposity measures and subclinical cardiovascular disease.
Keywords: African Americans, adiposity, calcified atherosclerotic plaque, cardiovascular disease, pericardial adipose tissue, type 2 diabetes
Introduction
Obesity, particularly abdominal adiposity, is associated with diabetogenic and atherogenic abnormalities that often lead to cardiovascular disease (CVD) in individuals with and without diabetes (1-5). Body mass index (BMI), waist circumference (WC), and volume of abdominal visceral adipose tissue (VAT) are related indices of cardio-metabolic risk (6-9) that independently predict CVD (10-14). The volume of pericardial adipose tissue (PAT) may constitute an especially harmful fat depot based on its location (15-20). Recent studies have shown that PAT is associated with CVD risk factors and CVD outcomes (15,21), associations persist after adjustment for other measures of adiposity (16,17). Ethnic differences have been reported in the volumes of abdominal VAT and subcutaneous adipose tissue (SAT). Relative to European American (EAs), African Americans (AAs) have less abdominal VAT but higher amounts of SAT, including after adjustment for BMI and total body fat (14,22,23). Whether differences in adiposity between AAs and EAs impact ethnic-specific rates of CVD are unknown.
Calcified atherosclerotic plaque in the coronary arteries and abdominal aorta are measures of subclinical CVD that predict future myocardial infarction and death (24,25). Despite more severe conventional cardio-metabolic risk factors in AAs relative to EAs, including albuminuria, and poorer glycemic, low-density lipoprotein (LDL) cholesterol, and hypertension control (26,27), AAs have markedly lower levels of calcified plaque (18,28). African ancestry appears to be protective from development of calcified plaque (29,30). Ethnic- and gender-specific analyses contrasting associations between calcified plaque in the aorta, coronary arteries, and carotid arteries with BMI, WC, PAT, VAT, and SAT have not been reported in patients with type 2 diabetes. The present analyses assessed relationships between anthropometric measures and adipose tissue volumes with subclinical CVD in the aorta, coronary, and carotid arteries in the full sample of high risk AA and EA Diabetes Heart Study (DHS) and African American-Diabetes Heart Study (AA-DHS) participants with type 2 diabetes (31).
Methods
Participants
Details of participant recruitment have been reported (28). EA and AA siblings concordant for type 2 diabetes were recruited from internal medicine clinics and community advertising in the DHS. In the DHS, diabetes was defined as a clinical diagnosis after the age of 34 years, with active insulin or hypoglycemic treatment and the absence of historical evidence of ketoacidosis. Unrelated AAs with type 2 diabetes were recruited in the follow-up AA-DHS using the same diagnostic criteria, except that type 2 diabetes was diagnosed after the age of 30 years (32). Both studies were approved by the Institutional Review Board at the Wake Forest School of Medicine. Participants provided written informed consent, and examinations were conducted in the Wake Forest Clinical Research Unit.
Clinical evaluation
Examinations included interviews for medical history and health behaviors, anthropometric measures, resting blood pressure, a fasting blood draw and a spot urine collection. Laboratory assays included urine albumin and creatinine, total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, glycated hemoglobin (HbA1c), fasting glucose, and blood chemistries. History of CVD was provided by self-report and medical record review, with emphasis on prior vascular procedures (coronary artery bypass grafting [CABG], coronary artery stenting, and coronary artery angioplasty).
Vascular imaging
Calcified plaque was measured in the coronary arteries (CAC), aorta, and carotid arteries using single and multidetector computed tomography (CT) systems incorporating a standardized scanning protocol based on those implemented in the National Heart Lung and Blood Institute’s MESA study, as reported (28,33). Traditionally, the Agatston score, also called the calcium score, has been used to report results. However, the nature of this scoring system adds noise to the CT measurement of calcified plaque compared to calcified plaque volume-based measures (34). This report used the calcium mass score (milligrams of calcium), which is derived from the volume score but also accounts for the density of calcified plaque on a pixel by pixel basis (35). Additional scoring parameters included a 90 Hounsfield unit (HU) threshold and two adjacent pixels to define the maximum calcified lesion size; the program accounted for slice thickness. Quantitative coronary artery calcium mass scores were excluded from analyses of severity in participants who had undergone prior CABG, coronary artery stenting, or coronary angioplasty.
Adipose tissue imaging
PAT, VAT, and SAT volumes were measured from volumetric CT acquisitions to reduce the variability related to slice location using Volume Analysis software (Advantage Windows Workstation, GE Healthcare, Waukesha, WI). As reported, a threshold of −190 to −30 HUs was used to define fat containing tissue (18,36).
Statistical methods
The observational and cross-sectional analyses in this report used SAS software (version 9.4, SAS Institute, Cary, NC). Sample means and standard deviations (SDs) were computed for continuous characteristics and proportions were calculated for discrete characteristics. For variables with highly skewed distributions, median values were reported to reflect the central tendency. The dependent variables were transformed to best approximate the normality assumption. The ethnicity comparison within men and women, respectively, was performed using the marginal model incorporating generalized estimating equations (GEEs). This model accounted for familial correlation using a sandwich estimator of the variance assuming exchangeable correlation.
The marginal model was fitted to compare measures of adiposity and calcified plaque between AAs and EAs, stratified by gender. To test whether the ethnicity effect on the adipose measures and calcified plaque was the same between men and women, an interaction between ethnicity and sex was additionally included in the model. Covariates included age, HbA1c, use of insulin or oral hypoglycemic agents, and prior CVD. Bonferroni corrections were applied to adjust for multiple comparison testing; p-values <0.01 (p=0.05/5) and <0.016 (p=0.05/3), respectively, were considered significant for association with adiposity and calcified plaque.
To assess associations between anthropometric measures and adipose tissue volumes with calcified plaque in AAs and EAs, two models were fitted separately because more than 20% of participants lacked calcified plaque in a given vascular bed (mass score=0). We initially modeled the probability of a positive score (presence/absence), and subsequently modeled the score given a calcified plaque score >0 (severity) in each vascular bed. The marginal models incorporating GEEs were used for both models. The outcomes included presence and severity of CAC, carotid artery calcified plaque, and aorta calcified plaque, and the covariates of interest included BMI, WC, PAT, VAT, and SAT. For relative comparisons, the covariates (adiposity measures) were standardized. For the initial model, the logit link and binomial distribution were fitted. The odds ratios (ORs) and their 95% confidence intervals (CIs) per SD increase in adiposity measures are reported. For the second model, the identity link and normal distribution were fitted. The regression coefficient estimates and their standard errors are reported. Age, gender, HbA1c, statins, smoking, prior CVD, hypertension, kidney disease (defined as an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2 and/or a urine albumin:creatinine ratio (UACR) >30mg/g), and C-reactive protein (CRP) were adjusted in the models. Analyses in AAs were additionally adjusted for African ancestry proportion based on ancestry informative markers, because African ancestry impacts calcified plaque (29,30). To test whether the associations between anthropometric measures/adipose tissue volumes and presence and severity of calcified plaque in AAs and EAs were the same, interactions between measures of adiposity with ethnicity were assessed. Interaction terms were removed from the model if non-significant, In addition, the gender interaction and 3-way interactions between adiposity, ethnicity, and gender were tested. Considering multiple comparisons, p-values <0.003 (p=0.05/15) were considered significant.
Results
A total of 1,315 individuals with type 2 diabetes were evaluated, 562 EAs from 444 families and 753 AAs from 644 families. Figure 1 displays a flowchart with participants in each analysis and sample sizes. Thirty participants lacked data on CAC, yielding 1,285 participants with evaluable CAC. An additional 184 participants who had prior coronary artery procedures were excluded from the analyses assessing the severity of CAC (31 AAs and 31 EAs with CABG; 84 AAs and 72 EAs with prior coronary artery angioplasty and/or stents [34 had both CABG and angioplasty/stent]). Another 169 study participants were not included in analyses assessing the severity of CAC due to absence of CAC (CAC mass score=0), for a total sample of 932 individuals in those analyses. Severity of carotid artery and aorta calcified plaque were evaluated in 830 and 1019 individuals, respectively. No participant had repair of an abdominal aortic aneurysm.
Figure 1. Participant enrollment flow chart, by ethnicity.
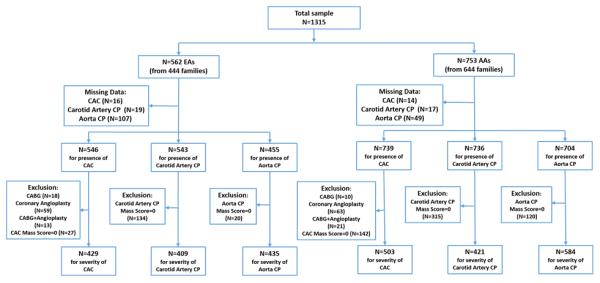
Abbreviations - EAs: European Americans; AAs: African Americans; CAC: coronary artery calcified atherosclerotic plaque; CP: calcified atherosclerotic plaque; CABG: coronary artery bypass grafting
Table 1 summarizes demographic and laboratory data, by gender and ethnicity. Compared to EA men and women, AA men and women were significantly younger. Mean diabetes durations were similar in both ethnic groups. Relative to EAs, AAs had higher diastolic blood pressures, HbA1c, LDL cholesterol, CRP, and eGFR. As expected, AAs had higher levels of HDL cholesterol and lower levels of triglycerides, both in men and women.
Table 1.
Demographic and laboratory characteristics of study participants, by ethnicity and gender
|
Men
|
Women
|
|||||
|---|---|---|---|---|---|---|
|
European American
(N=249) |
African American
(N=303) |
P-value † |
European American
(N=313) |
African American
(N=450) |
P-value † | |
| Age, years | 62.8 (9.1) | 56.6 (9.6) | <0.001 | 61.8 (8.8) | 56.3 (9.5) | <0.001 |
| Smoking | <0.001 | 0.10 | ||||
| Never | 59 (23.7) | 83 (27.4) | 177 (56.9) | 219 (49.0) | ||
| Former | 157 (63.1) | 128 (42.2) | 88 (28.3) | 144 (32.2) | ||
| Current | 33 (13.3) | 92 (30.4) | 46 (14.8) | 84 (18.8) | ||
| Diabetes duration, years | 10.8 (7.6) | 10.2 (7.8) | 0.11* | 9.5 (6.5) | 10.1 (7.4) | 0.34* |
| Median (Min- Max) | 9 (1-46) | 8 (0-52) | 7 (0-35) | 8 (0-48) | ||
| Weight, kg | 98.3(19.2) | 100.5(23.6) | 0.16 | 86.9(19.7) | 97.9(24.3) | <0.001 |
| Systolic blood pressure, mmHg | 139.2 (18.7) | 133.5 (18.3) | <0.001 | 139.7 (18.0) | 136.4 (21.1) | 0.02 |
| Diastolic blood pressure, mmHg | 74.4 (10.1) | 78.4 (10.9) | <0.001 | 71.7 (9.7) | 76.3 (11.8) | <0.001 |
| Hypertension, n (%) | 209 (83.9) | 245 (80.9) | 0.40 | 275 (87.9) | 385 (85.6) | 0.22 |
| Insulin therapy, n (%) | 58 (23.3) | 125 (41.3) | 0.02 | 80 (25.6) | 180 (40.1) | <.001 |
| Oral hypoglycemic agents, n (%) | 205 (82.3) | 227 (74.9) | 0.12 | 239 (76.4) | 348 (77.5) | 0.14 |
| Statins, n (%) | 113 (45.4) | 138 (45.5) | 0.85 | 121 (38.7) | 209 (46.4) | 0.005 |
| Prior CVD, n (%) | 110 (44.9) | 95 (32.8) | 0.04 | 89 (28.9) | 129 (29.3) | 0.75 |
| Total Cholesterol, mmol/L | 4.52 (0.93) | 4.52 (0.86) | 0.97 | 5.06 (1.15) | 4.96 (1.03) | 0.50 |
| HDL-cholesterol, mmol/L | 0.99 (0.26) | 1.15 (0.31) | <0.001 | 1.21 (0.32) | 1.31 (0.38) | <0.001 |
| LDL-cholesterol, mmol/L | 2.56 (0.77) | 2.74 (0.96) | 0.03 | 2.81 (0.91) | 2.89 (0.94) | 0.30 |
| Triglycerides, mmol/L | 2.27 (−1.62) | 1.54(1.75) | <0.001* | 2.31 (1.32) | 1.44 (1.05) | <.001* |
| Median (Min- Max) | 1.84 (0.34-12.07) | 1.20 (0.23-20.88) | 2.05 (0.53-12.03) | 1.16 (0.43-12.31) | ||
| HbA1c, % | 7.5 (1.6) | 8.3 (2.0) | <0.001 | 7.4 (1.6) | 8.3 (2.4) | <0.001 |
| Fasting glucose, mmol/L | 8.05 (2.96) | 8.77 (4.16) | 0.11* | 8.11 (2.96) | 8.33 (3.67) | 0.91* |
| Median (Min-Max) | 133 (38-377) | 138.5 (32-568) | 134 (66-436) | 134 (32-448) | ||
| CKD-EPI eGFR, ml/min/1.73m2 | 69.8 (15.0) | 87.2 (22.7) | <0.001 | 64.5 (16.2) | 84.9 (25.9) | <0.001 |
| Urine albumin:creatinine, mg/mmol | 9.99 (28.20) | 17.98 (58.10) | 0.38* | 10.74 (62.14) | 21.87 (80.64) | 0.58* |
| Median (Min-Max) | 1.71 (0.08-230.48) | 2.10 (0-502.43) | 1.49 (0.08-1003.78) | 1.36 (0-755.23) | ||
| C-reactive protein, nmol/L | 38.10 (47.62) | 66.67 (95.24) | <0.001* | 66.67 (95.24) | 104.76 (180.90) | <0.001* |
| Median (Min-Max) | 19.05 (0.10-285.72) | 28.57 (0-651.44) | 38.10 (0.95-914.30) | 57.14 (0-1895.28) | ||
Data expressed as mean (SD) for continuous characteristics; n (%) for discrete characteristics; median (Min - Max) for variables with highly skewed distributions. CVD: cardiovascular disease; CKD-EPI eGFR: Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate; HbA1c: hemoglobin A1c.
Unadjusted GEE model used to calculate p-values.
Log transformed variables used to calculate p-values.
Gender-specific ethnic differences in measures of adiposity and calcified plaque mass scores are displayed in Table 2, adjusted for age, HbA1c, prior CVD, and use of insulin and oral hypoglycemic agents. AA women had significantly higher WC and BMI than EA women. AA men had a significantly lower WC than EA men, with similar BMIs. Despite equivalent or higher BMIs, AA men and women had lower volumes of VAT and PAT than EA men and women (both p<0.001). Relative to EA men, AA men also had a lower SAT; however, this effect did not meet the Bonferroni correction threshold. The ethnicity effect differed between men and women for WC, PAT and VAT (ethnicity-gender interaction p-values <0.01). The percentage of participants with absence of calcified plaque in the three vascular beds was higher in AAs than in EAs, and in women relative to men (Table 2). Because distributions of calcified plaque were highly skewed, median values reported in Table 2 are more reflective of central tendency. Compared to EA men, AA men had significantly lower levels of CAC, carotid artery, and aorta calcified plaque, particularly CAC (p-value ≤0.001). Among women, AAs had lower CAC than EAs (p=0.010). The ethnicity effect differed between men and women for CAC and carotid artery calcified plaque (interaction p-value ≤0.002).
Table 2.
Gender-specific adiposity and calcified atherosclerotic plaque comparisons in African Americans and European Americans
|
Men
|
Women
|
Ethnicity
Interaction P-value |
|||||
|---|---|---|---|---|---|---|---|
| European American (n=249) | African American (n=303) | P† | European American (n=313) | African American (n=450) | P† | ||
| Anthropometry/adipose volumes | |||||||
| Body mass index, mean (SD) | 31.5 (5.5) | 32.2 (7.2) | 0.51 | 33.1 (7.0) | 37.0 (8.8) | <0.001 | <0.001 |
| Waist circumference (cm)* | 110.1 (15.2) | 108.7 (17.3) | 0.006 | 105.4 (17.5) | 112.6 (19.4) | 0.003 | <0.001 |
| Median (Min-Max) | 107.8 (78.0-220.0) | 106.5 (69.0-182.0) | 105.0(63.5-195.0) | 110.3 (73.0-273.3) | |||
| PAT volume* | 148.3 (61.7) | 93.2 (45.7) | <0.001 | 118.5 (45.5) | 84.0 (32.7) | <0.001 | 0.015 |
| Median (Min-Max) | 137.5 (31.4-333.7) | 82.8 (24.0-285.3) | 110.3 (19.6-307.8) | 80.8 (18.9-219.3) | |||
| VAT volume* | 313.1 (164.6) | 181.6 (83.3) | <0.001 | 275.7 (150.9) | 174.6 (64.1) | <0.001 | 0.037 |
| Median (Min-Max) | 284.0 (50.8-982.2) | 170.7 (16.3-470.3) | 236.6(28.3-860.3) | 168.4 (31.6-379.6) | |||
| SAT volume* | 363.5 (199.2) | 334.4 (156.3) | 0.041 | 484.3 (267.8) | 500.6 (170.5) | 0.10 | 0.004 |
| Median (Min-Max) | 308.4 (40.2-1048.5) | 316.6 (45.8-796.1) | 432.8(16.4-1704.1) | 484.6(108.0-915.7) | |||
|
| |||||||
| Calcified plaque (CP) | |||||||
| Coronary artery CP*,** | 2095.7 (3811.8) | 678.0 (1460.9) | <0.001 | 469.5 (1158.2) | 397.6 (1101.4) | 0.010 | <0.001 |
| Median (Min-Max) | 622.8 (0-25420.0) | 75.5 (0-11549.0) | 73.5 (0-10317.5) | 18.5 (0-9762.0) | |||
| Absent (CAC=0) | 8 (3.31%) | 59 (19.9%) | <0.001 | 20 (6.6%) | 90 (20.3%) | <0.001 | 0.21 |
| Carotid artery CP* | 491.3 (896.7) | 210.0 (614.3) | 0.001 | 184.9 (560.1) | 152.1 (468.7) | 0.60 | 0.002 |
| Median (Min-Max) | 94.0 (0-5604.5) | 9.3 (0-6703.5) | 14.5 (0-6121.5) | 3.0 (0-4987.5) | |||
| Absent (CP=0) | 46 (19.0%) | 120 (40.5%) | 0.006 | 88 (29.2%) | 195 (44.3%) | 0.08 | 0.13 |
| Aorta CP* | 14487 (18746) | 5782 (11512) | <0.001 | 6923 (10344) | 5137 (9327) | 0.08 | 0.11 |
| Median (Min-Max) | 6895 (0-94156) | 1181(0-91886) | 2700 (0-57165) | 828 (0-67017) | |||
| Absent (CP=0) | 7 (3.4%) | 38 (13.2%) | 0.05 | 13 (5.2%) | 82 (19.7%) | 0.009 | 0.80 |
Data expressed as mean (SD) and median (Min - Max). PAT: pericardial adipose tissue; VAT: visceral adipose tissue; SAT: subcutaneous tissue; CP: calcified atherosclerotic plaque.
GEE model used, adjusted for age, HbA1c, use of insulin or oral hypoglycemic agents, and prior CVD. Bolded p-values pass Bonferroni correction: anthropometry/adipose volumes analysis threshold p<0.010 (0.05/5); calcified plaque gender-specific analysis threshold p<0.016 (0.05/3).
Log transformed variable used to calculate p-values.
Excluding those who underwent coronary artery bypass grafting (31 AAs; 31 EAs), prior stenting and coronary angioplasty (84 AAs; 72 EAs).
Table 3 displays associations between measures of adiposity and presence and severity of calcified plaque, by ethnicity. In multivariable analyses adjusting for age, gender, level of education, HbA1c, statins, smoking, prior CVD, hypertension, kidney disease, African ancestry proportion, and CRP, PAT was the only adiposity measure with a significant positive association with the presence of CAC (OR [95%CI]: 1.94 [1.36, 2.75] per SD increase, p<0.001) in AAs. This association was not observed in EAs (OR [95%CI]: 0.91 [0.58, 1.43] per SD increase, p=0.68), yielding an interaction p=0.007. Significant relationships between ethnicity and adiposity measures were not observed between PAT and either carotid artery or aorta calcified plaque.
Table 3.
Association of measures of adiposity with presence and severity of calcified atherosclerotic plaque, by ethnicity
| African Americans | European Americans | Ethnicity Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Presence | Severity* | Presence | Severity* | P value | ||||||
| OR (95% CI) | P value | Estimate (SE) | P value | OR (95% CI) | P value | Estimate (SE) | P value | Presence | Severity | |
| Coronary | N=739 | N=503 | N=546 | N=429 | ||||||
| BMI | 1.21 (0.94,1.55) | 0.13 | −0.01 (0.10) | 0.92 | 0.78 (0.47,1.30) | 0.35 | 0.06 (0.13) | 0.65 | 0.09 | 0.55 |
| WC | 1.25 (0.98,1.60) | 0.07 | −0.03 (0.10) | 0.75 | 0.83 (0.60,1.14) | 0.24 | 0.19 (0.10) | 0.047 | 0.06 | 0.05 |
| PAT | 1.94 (1.36,2.75) | <0.001 | 0.08 (0.13) | 0.53 | 0.91 (0.58,1.43) | 0.68 | 0.06 (0.10) | 0.58 | 0.007 | 0.86 |
| VAT | 1.49 (0.99,2.25) | 0.06 | −0.26 (0.18) | 0.13 | 0.80 (0.57,1.13) | 0.20 | 0.02 (0.10) | 0.81 | 0.018 | 0.11 |
| SAT | 1.16 (0.85,1.57) | 0.35 | −0.02 (0.13) | 0.90 | 0.73 (0.52,1.02) | 0.06 | −0.04 (0.09) | 0.61 | 0.036 | 0.41 |
| Carotid | N=736 | N=421 | N=543 | N=409 | ||||||
| BMI | 0.87 (0.73,1.04) | 0.14 | 0.01 (0.11) | 0.92 | 0.96 (0.68,1.36) | 0.83 | 0.09 (0.16) | 0.59 | 0.50 | 0.57 |
| WC | 0.86 (0.72,1.04) | 0.13 | 0.01 (0.12) | 0.96 | 1.04 (0.79,1.37) | 0.79 | 0.04 (0.12) | 0.70 | 0.17 | 0.40 |
| PAT | 0.87 (0.67,1.12) | 0.28 | −0.02 (0.13) | 0.87 | 0.91 (0.71,1.17) | 0.48 | −0.06 (0.09) | 0.53 | 0.62 | 0.77 |
| VAT | 0.65 (0.47,0.91) | 0.012 | −0.20 (0.19) | 0.30 | 1.02 (0.81,1.28) | 0.89 | −0.09 (0.07) | 0.22 | 0.019 | 0.40 |
| SAT | 0.78 (0.61,1.01) | 0.06 | 0.13 (0.14) | 0.36 | 0.93 (0.73,1.19) | 0.56 | −0.02 (0.09) | 0.78 | 0.34 | 0.37 |
| Aorta | N=704 | N=584 | N=455 | N=435 | ||||||
| BMI | 0.77 (0.61,0.98) | 0.035 | −0.24 (0.09) | 0.009 | 1.80 (0.89,3.63) | 0.10 | −0.33 (0.13) | 0.011 | 0.05 | 0.53 |
| WC | 0.75 (0.57,0.97) | 0.032 | −0.31 (0.10) | 0.002 | 1.70 (0.96,3.00) | 0.07 | −0.32 (0.09) | <0.001 | 0.09 | 0.52 |
| PAT | 0.88 (0.62,1.25) | 0.46 | −0.001 (0.13) | 0.99 | 1.41 (0.72,2.75) | 0.32 | −0.14 (0.09) | 0.13 | 0.36 | 0.70 |
| VAT | 0.70 (0.43,1.14) | 0.16 | −0.48 (0.19) | 0.012 | 1.46 (0.68,3.14) | 0.33 | −0.10 (0.07) | 0.14 | 0.23 | 0.026 |
| SAT | 0.62 (0.44,0.88) | 0.007 | −0.39 (0.12) | 0.001 | 1.16 (0.67,2.00) | 0.59 | −0.17 (0.09) | 0.04 | 0.048 | 0.49 |
Estimates are per standard deviation (in the full cohort) increment: 7.8 kg/m2 of BMI, 17.9 cm of WC, 51.6 cm3 of PAT, 130.6 cm3 of VAT, 213.2 cm3 of SAT. Adjusted for age, gender, level of education, HbA1c, statins, smoking, prior CVD, hypertension, kidney disease (eGFR<60 ml/min/1.73m2 or urine albumin:creatinine >30 mg/g), and C-reactive protein. Additional adjustment for African ancestry proportion performed in African Americans.
Participants who underwent coronary artery bypass grafting (31 AAs; 31 EAs), prior stenting and/or coronary angioplasty (84 AAs; 72 EAs) were excluded from severity of CAC analyses. BMI: body mass index; WC: waist circumference; PAT: pericardial adipose tissue; VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue. N=sample size. Bolded p-values pass Bonferroni correction threshold of p<0.003 (0.05/15).
Inverse associations were observed between SAT and severity of aorta calcified plaque (p=0.001) in AAs. None of the gender interactions or 3-way interactions was statistically significant (data not shown). Association analyses were repeated in AAs without adjustment for African ancestry proportion; results were consistent with those in the ancestry-adjusted model.
Because the majority of interactions between ethnicity and adiposity were non-significant (Table 3), associations between adiposity and calcified plaque were evaluated in the full sample (Table 4). Significant inverse associations between BMI, WC, and SAT with severity of aorta calcified plaque were observed in the full sample (all p<0.002); an inverse association was also present between VAT and aorta calcified plaque (p=0.02); however, this did not remain significant considering stringent correction for multiple testing (p<0.003). As expected, the PAT association with presence of CAC was weaker due to the different directions of association in AAs and EAs; stratified analyses are more relevant for this comparison.
Table 4.
Association of adiposity measures with calcified atherosclerotic plaque, full sample
| Full sample | ||||
|---|---|---|---|---|
| Presence | Severity | |||
| Calcified Plaque | OR (95% CI) | P-value | Estimate (SE) | P-value |
| Coronary Artery* | ||||
| BMI | 1.15 (0. 93,1.43) | 0.21 | 0.06 (0.08) | 0.41 |
| WC | 1.18 (0.95,1.45) | 0.13 | 0.08 (0.07) | 0.27 |
| PAT | 1.49 (1.10,2.03) | 0.011 | 0.06 (0.08) | 0.43 |
| VAT | 1.14 (0.82,1.57) | 0.44 | −0.001 (0.08) | 0.99 |
| SAT | 0.98 (0.76,1.27) | 0.88 | −0.01 (0.07) | 0.89 |
| Carotid Artery | ||||
| BMI | 0.90 (0.77,1.04) | 0.16 | 0.04 (0.08) | 0.61 |
| WC | 0.93 (0.80,1.07) | 0.31 | 0.02 (0.08) | 0.78 |
| PAT | 0. 90 (0.75,1.07) | 0.22 | −0.04 (0.07) | 0.56 |
| VAT | 0.92 (0.78,1.09) | 0.35 | −0.11 (0.07) | 0.11 |
| SAT | 0.89 (0.75,1.04) | 0.15 | −0.003 (0.07) | 0.97 |
| Aorta | ||||
| BMI | 0.86 (0.69,1.06) | 0.16 | −0.24 (0.08) | 0.002 |
| WC | 0.87 (0.71,1.07) | 0.19 | −0.28 (0.07) | <0.001 |
| PAT | 0.98 (0.74,1.31) | 0.92 | −0.04 (0.07) | 0.54 |
| VAT | 0.99 (0.73,1.36) | 0.97 | −0.15 (0.07) | 0.023 |
| SAT | 0.81 (0.63,1.03) | 0.09 | −0.21 (0.07) | 0.002 |
Estimates are per standard deviation increment in the full cohort: 7.8 kg/m2 BMI, 17.9 cm WC, 51.6 cm3 PAT, 130.6 cm3 VAT, and 213.2 cm3 SAT. Results were adjusted for age, gender, level of education, HbA1c, statins, smoking, prior CVD, hypertension, kidney disease, and C-reactive protein.
Participants who underwent coronary artery bypass grafting (31 AAs; 31 EAs), prior stenting and/or coronary angioplasty (84 AAs; 72 EAs) were excluded from severity of CAC analyses. BMI: body mass index; WC: waist circumference; PAT: pericardial adipose tissue; VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue. Bolded p-values pass Bonferroni correction threshold of p<0.003 (0.05/15).
Discussion
Marked ethnic and gender differences are present in BMI, WC, adipose tissue volumes, and calcified plaque in AAs and EAs with type 2 diabetes. Despite equivalent or higher BMIs, AA men and women had significantly lower volumes of VAT and PAT than EA men and women. In spite of AAs having generally adverse cardio-metabolic risk factor profiles relative to EAs, along with higher WC and BMI (especially in women), AAs had paradoxically lower levels of CAC (p=0.010), with a trend toward lower aorta calcified plaque (p=0.08). In AAs, PAT was the only measure of adiposity with a statistically significant positive association with presence of CAC, this relationship differed from that in EAs. No other adipose volume or anthropometric measure was positively associated with CAC in EAs with type 2 diabetes. Particularly striking was the complete absence of positive associations between non-PAT measures of adiposity and calcified plaque in the coronary, carotid, or aortic vascular beds; in fact, several significant negative associations were detected. As such, volume of PAT appears to be the most potent factor associated with CAC in AAs with type 2 diabetes; BMI, WC, SAT, and VAT did not display significant relationships with CAC in EAs and AAs with type 2 diabetes. The direct relationship between PAT and CAC in AAs may contribute to lower rates of myocardial infarction in AAs with equivalent access to healthcare as EAs (37,38).
Paradoxical inverse associations were often present between non-PAT measures of adiposity and calcified plaque. BMI, WC, and SAT were inversely associated with the severity of aorta calcified plaque in a combined analysis of AAs and EAs. These consistent inverse associations between measures of adiposity and calcified plaque in multiple vascular distributions were unanticipated. Results are important to clinicians, because they may equate adiposity measures such as BMI, WC, and SAT with equivalent risk for subclinical CVD in patients with type 2 diabetes. The paradoxical findings support a focus limited to PAT for risk of coronary atherosclerosis, rather than on BMI, WC, or volumes of abdominal VAT or SAT.
Positive associations between PAT and CAC have been observed in prior reports from AA-DHS (18), and the MESA (19), Framingham Heart Study (FHS) (15), and Jackson Heart Study (JHS) (20) which were not enriched for participants with type 2 diabetes. MESA studied 6,814 Caucasian, African American, Chinese American, and Hispanic adult participants without prior CVD (19). MESA found that PAT was positively associated with the presence and severity of CAC; associations did not vary based on ethnicity. In the FHS, 1,155 participants free of prior CVD were examined (15). After accounting for metabolic risk factors, FHS reported that PAT was positively associated with the presence of CAC (15). The JHS also reported a significant positive relationship between PAT and presence of CAC, but not with aorta calcified plaque in a community-based cohort of 1,414 AAs (20). Approximately 15% of JHS participants had diabetes.
The mechanism(s) behind inverse relationships between aorta calcified plaque with multiple non-PAT measures of adiposity is unknown. Participants in this report were at higher risk for CVD than those in MESA, FHS, and JHS because they all had type 2 diabetes and participants with prior CVD were enrolled. The present report demonstrated the expected positive association between PAT and CAC in AAs, but not in EAs. We hypothesize that the approximate ten year mean diabetes durations in participants in the present study increased levels of subclinical atherosclerosis in all three vascular beds, compared to studies in lower risk population-based samples (39). EAs (with and without diabetes) are also known to have markedly higher levels of calcified plaque than AAs (40). Thus, it is possible that diabetes-induced increases in calcified plaque might have contributed to the unexpected inverse relationships with adiposity measures in this high risk sample. It must be noted that CAC does not reflect the burden of “soft plaque”, likely more dangerous regarding risk for CVD. As such, CAC does not reflect total atherosclerotic burden in an individual and some participants may have had lower levels of CAC despite more soft plaque (and vice versa). MESA, JHS, and FHS have not reported associations between CAC, carotid artery and aorta calcified plaque with simultaneous measures of VAT and SAT. Thus, the present results extend prior analyses focused on PAT and CAC. Together, DHS and JHS demonstrate that positive association between PAT and CAC in AAs is similar in individuals with and without diabetes (20).
The biomolecular mechanisms linking PAT volumes to CAC remain under study. Greif et al. (21) demonstrated that low adiponectin and low HDL-cholesterol levels and greater degrees of inflammation (higher CRP and levels of tumor necrosis factor [TNF]) were present with higher volumes of PAT. This suggests that local inflammation in PAT could potentiate vascular calcification, or that local atherosclerosis and inflammation in the coronary arteries could promote local adipose accumulation. In contrast, PAT was not associated with aorta and carotid artery calcified plaque in this type 2 diabetes-affected cohort (Table 3); this supports the hypothesis that PAT influences atherosclerosis in the adjacent coronary vasculature with a local effect.
The present study has several strengths and some limitations. A relatively large sample of AAs and EAs with ten year mean durations of diabetes was carefully evaluated; this permitted direct comparisons between ethnic groups. PAT, VAT, and SAT were determined by multi-slice CT providing precise volumetric measures; subclinical atherosclerosis in the coronary arteries, carotid arteries, and aorta were simultaneously assessed to provide assessments of subclinical CVD beyond CAC. In contrast to highly selected populations at low risk for CVD (those lacking diabetes or prior CVD events), this report assessed PAT, VAT, SAT, and other anthropometric measures for association with calcified plaque in three vascular distributions in a general type 2 diabetes-affected patient population. Inverse associations were consistently detected between conventional measures of adiposity with aorta calcified plaque in AAs and EAs. Other studies may not have identified these relationships if they focused primarily on CAC. Although AAs were younger than EAs, mean and median durations of type 2 diabetes were nearly equivalent between age- and gender- groups (Table 1) because diabetes onset is typically earlier in AAs. It is unlikely that the younger age of AAs impacted levels of calcified plaque, as markedly lower levels of subclinical atherosclerosis are observed in individuals with recent African ancestry, relative to European (28,40). As stated, a limitation of this and other reports is the failure of CAC to reflect total atherosclerotic burden. The findings in this report are specifically relevant to populations with type 2 diabetes, and we cannot generalize relationships in other populations. We were also unable to perform ethnicity-by-gender analyses between PAT and CAC in each ethnicity-gender-group due to an insufficient sample size (the model failed to converge). However, interaction effects based on gender were tested across the ethnic groups for relationships between all measures of adiposity and presence of CAC; significant effects were not detected. The cross-sectional nature of this study limits our conclusions regarding causality of association. We lack data on income, but included education as a surrogate for socioeconomic status. Our conclusions generally held when considering multiple comparisons. When a stringent Bonferroni correction was applied to account for multiple testing (alpha=0.05/15=0.003, based on the five adiposity and three calcified plaque measures); only PAT remained positively associated with CAC in AAs (p<0.001). In addition, the inverse associations between SAT and severity of aorta calcified plaque (p=0.001) in AAs remained statistically significant, as did several of the inverse relationships between measures of adiposity and aorta calcified plaque (p<0.003). We note that interpretations are dependent on the stringency of the correction that is applied. PAT remained the only measure of adiposity that was significantly and positively associated with CAC in AAs; all remaining significant associations with adiposity measures remained in the negative direction.
Beyond reproducing the known positive association between PAT and CAC in AAs, we determined that non-PAT measures of adiposity were not reliably positively associated with the presence or severity of subclinical atherosclerosis in patients with type 2 diabetes. Despite widespread use of WC, BMI, SAT, and VAT to estimate obesity-associated CVD risk in patients with type 2 diabetes, PAT appears to be superior regarding its association with subclinical coronary artery disease. Many non-PAT measures of adiposity appeared to be inversely associated with calcified plaque in the aorta and had a trend toward inverse association with carotid artery calcified plaque; these were paradoxical relationships identified in high-risk diabetes-affected participants and may reflect nutritional or hyperglycemia-mediated effects different from those in individuals lacking diabetes. Gender- and ethnicity-based differences in adipose tissue distributions were detected and should be considered when applying established anthropometric and adipose volume measures to association with subclinical CVD in AAs and EAs with longstanding diabetes.
What is known about this topic?
-
-
Adiposity independently predicts risk for cardiovascular disease in population-based studies.
-
-
Relationships between measures of adiposity (body mass index, waist circumference, and volumes of visceral, subcutaneous, and pericardial adipose tissue) with subclinical cardiovascular disease are less clear in high risk populations, such as in those with type 2 diabetes.
What does this report add?
-
-
This report assessed relationships between simultaneously measured calcified atherosclerotic plaque in the aorta, coronary and carotid arteries with BMI, waist circumference, and computed tomography-determined volumes of visceral, subcutaneous, and pericardial adipose tissue in a large sample of African Americans and European Americans with type 2 diabetes.
-
-
Pericardial adipose tissue volume was positively associated with coronary artery calcified plaque in African Americans, but not in European Americans.
-
-
Paradoxical inverse associations were detected between aorta calcified atherosclerotic plaque and several non-pericardial adipose tissue adiposity measures, including BMI, waist circumference, and volume of subcutaneous adipose tissue. Relative to population-based studies, relationships between measures of adiposity and subclinical cardiovascular disease appear to differ in individuals with type 2 diabetes.
Acknowledgments
The authors thank study participants and research coordinators Cassandra Bethea, Benita Bowman, and Benjamin Bagwell. The results presented in this paper have not been published previously in whole or part, except in abstract format. No author reports a conflict of interest related to this work. Grant support included General Clinical Research Center of Wake Forest School of Medicine M01 RR07122; NIH RO1 DK071891 (BIF); AR48797 (JJC); and HL67348 (DWB).
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
The authors report no conflicts of interest in the performance of this work.
References
- 1.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 2.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 4.Gaillard T. Consequences of Abdominal Adiposity within the Metabolic Syndrome Paradigm in Black People of African Ancestry. J Clin Med. 2014;3:897–912. doi: 10.3390/jcm3030897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JD, Borel AL, Nazare JA, et al. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA study. J Clin Endocrinol Metab. 2012;97:1517–1525. doi: 10.1210/jc.2011-2550. [DOI] [PubMed] [Google Scholar]
- 6.Kahn HS, Bullard KM. Beyond Body Mass Index: Advantages of Abdominal Measurements for Recognizing Cardiometabolic Disorders. Am J Med. 2015 doi: 10.1016/j.amjmed.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 8.Katzmarzyk PT, Heymsfield SB, Bouchard C. Clinical utility of visceral adipose tissue for the identification of cardiometabolic risk in white and African American adults. Am J Clin Nutr. 2013;97:480–486. doi: 10.3945/ajcn.112.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bener A, Yousafzai MT, Darwish S, Al-Hamaq AO, Nasralla EA, Abdul-Ghani M. Obesity index that better predict metabolic syndrome: body mass index, waist circumference, waist hip ratio, or waist height ratio. J Obes. 2013;2013:269038. doi: 10.1155/2013/269038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75:683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 11.Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah RV, Murthy VL, Abbasi SA, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–1235. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 16.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–154. doi: 10.1016/j.atherosclerosis.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Divers J, Wagenknecht LE, Bowden DW, et al. Ethnic differences in the relationship between pericardial adipose tissue and coronary artery calcified plaque: African-American-diabetes heart study. J Clin Endocrinol Metab. 2010;95:5382–5389. doi: 10.1210/jc.2010-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClain J, Hsu F, Brown E, et al. Pericardial adipose tissue and coronary artery calcification in the Multi-ethnic Study of Atherosclerosis (MESA) Obesity (Silver Spring) 2013;21:1056–1063. doi: 10.1002/oby.20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Fox CS, Hickson D, et al. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes Care. 2010;33:1635–1639. doi: 10.2337/dc10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greif M, Leber AW, Saam T, et al. Determination of pericardial adipose tissue increases the prognostic accuracy of coronary artery calcification for future cardiovascular events. Cardiology. 2012;121:220–227. doi: 10.1159/000337083. [DOI] [PubMed] [Google Scholar]
- 22.Katzmarzyk PT, Bray GA, Greenway FL, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Coady S, Carr JJ, Hoffmann U, Taylor HA, Fox CS. Differential associations of abdominal visceral, subcutaneous adipose tissue with cardiometabolic risk factors between African and European Americans. Obesity (Silver Spring) 2014;22:811–818. doi: 10.1002/oby.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 25.Wong ND, Hsu JC, Detrano RC, Diamond G, Eisenberg H, Gardin JM. Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol. 2000;86:495–498. doi: 10.1016/s0002-9149(00)01000-6. [DOI] [PubMed] [Google Scholar]
- 26.Clark LT, Ferdinand KC, Flack JM, et al. Coronary heart disease in African Americans. Heart Dis. 2001;3:97–108. doi: 10.1097/00132580-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Despres JP, Couillard C, Gagnon J, et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20:1932–1938. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 28.Freedman BI, Hsu FC, Langefeld CD, et al. The impact of ethnicity and sex on subclinical cardiovascular disease: the Diabetes Heart Study. Diabetologia. 2005;48:2511–2518. doi: 10.1007/s00125-005-0017-2. [DOI] [PubMed] [Google Scholar]
- 29.Wassel CL, Pankow JS, Peralta CA, Choudhry S, Seldin MF, Arnett DK. Genetic ancestry is associated with subclinical cardiovascular disease in African-Americans and Hispanics from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Genet. 2009;2:629–636. doi: 10.1161/CIRCGENETICS.109.876243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Divers J, Palmer ND, Lu L, et al. Admixture mapping of coronary artery calcified plaque in African Americans with type 2 diabetes mellitus. Circ Cardiovasc Genet. 2013;6:97–105. doi: 10.1161/CIRCGENETICS.112.964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowden DW, Cox AJ, Freedman BI, et al. Review of the Diabetes Heart Study (DHS) family of studies: a comprehensively examined sample for genetic and epidemiological studies of type 2 diabetes and its complications. Rev Diabet Stud. 2010;7:188–201. doi: 10.1900/RDS.2010.7.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Divers J, Wagenknecht LE, Bowden DW, et al. Ethnic differences in the relationship between albuminuria and calcified atherosclerotic plaque: the African American-diabetes heart study. Diabetes Care. 2010;33:131–138. doi: 10.2337/dc09-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 34.Budoff MJ, McClelland RL, Chung H, et al. Reproducibility of coronary artery calcified plaque with cardiac 64-MDCT: the Multi-Ethnic Study of Atherosclerosis. AJR Am J Roentgenol. 2009;192:613–617. doi: 10.2214/AJR.08.1242. [DOI] [PubMed] [Google Scholar]
- 35.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208:807–814. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- 36.Wheeler GL, Shi R, Beck SR, et al. Pericardial and visceral adipose tissues measured volumetrically with computed tomography are highly associated in type 2 diabetic families. Invest Radiol. 2005;40:97–101. doi: 10.1097/00004424-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26:2392–2399. doi: 10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- 38.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal S, Cox AJ, Herrington DM, et al. Coronary calcium score predicts cardiovascular mortality in diabetes: diabetes heart study. Diabetes Care. 2013;36:972–977. doi: 10.2337/dc12-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]


