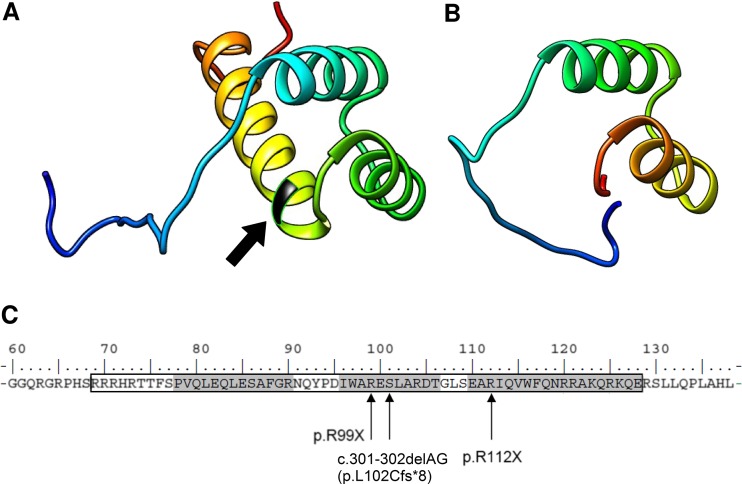
Fig. 5.
Modelling of the PROP1 homeodomain (AA 69–128, framed). a Structural model of the PROP1 homeodomain with depicted position of 112 arginine (arrow). b Prediction of the effect of R112X on the structure, revealing the lack of the third, longest alpha helix folding. c A string of the selected region in the PROP1 protein sequence encompassing the homeodomain (framed) with three alpha-helix folding (shaded) and marked novel mutation site. The mutations lead to a similar phenotype p.R99X, c.301-302delAG (p.L102Cfs*8, also referred to as p.S109X)