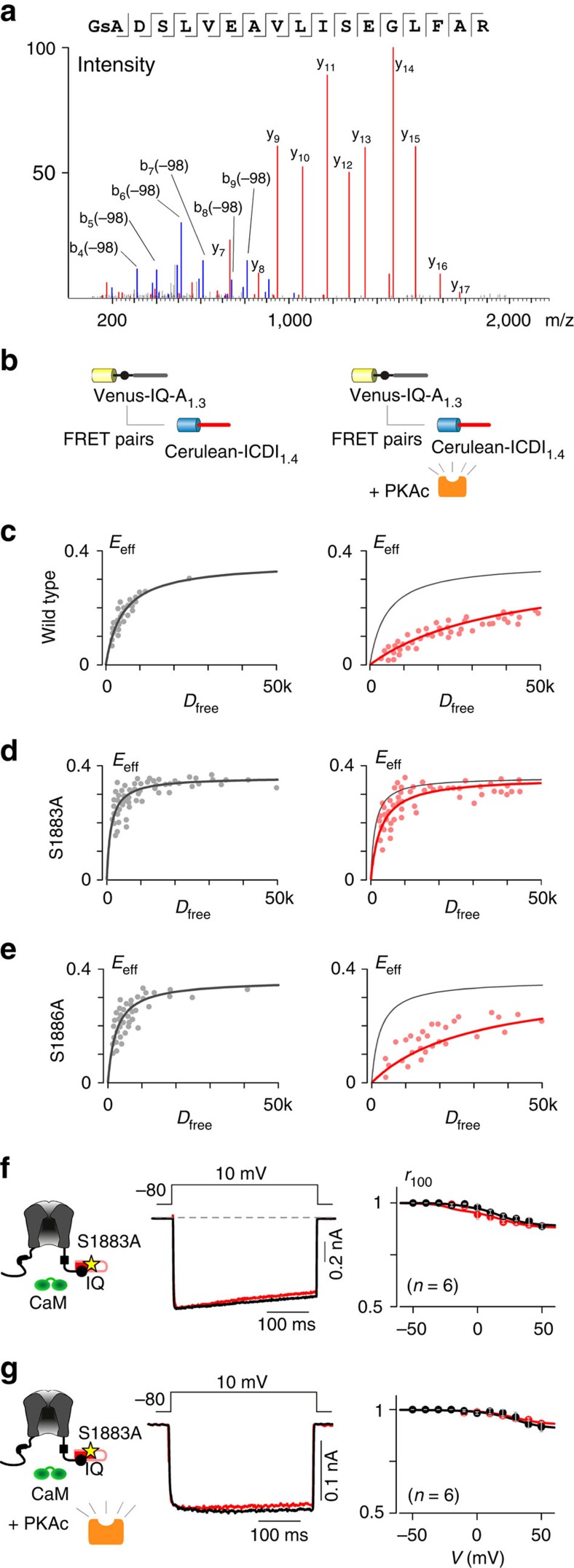
Figure 6. Identification of the PKA phosphorylation site within ICDI1.4.
(a) The mass spectra of the ICDI1.4 peptide, showing phosphorylation at S1883. (b) Flow-cytometric FRET binding assays (c–e) between Venus-IQ-A1.3 and Cerulean-ICDI1.4 demonstrating the modulation of binding due to PKAc overexpression. (c) Overexpression of PKAc reduced binding between IQ-A1.3 and wild-type ICDI1.4. (d) Overexpression of PKAc did not affect binding between IQ-A1.3 and ICDI1.4 harboring an S1883A mutation. (e) Overexpression of PKAc reduced binding between IQ-A1.3 and ICDI1.4 harbouring an S1886A mutation. (f) Similar to wild-type CaV1.3S/1.4DCT channels (Fig. 4c), CaV1.3S/1.4DCT channels containing the S1883A mutation had minimal CDI. (g) Unlike wild-type CaV1.3S/1.4DCT (Fig. 4d), CaV1.3S/1.4DCT channels with the S1883A mutation did not exhibit increased CDI with overexpression of PKAc.