Abstract
We developed an Australian database for the identification of Aspergillus, Scedosporium, and Fusarium species (n = 28) by matrix-assisted laser desorption ionization−time of flight mass spectrometry (MALDI-TOF MS). In a challenge against 117 isolates, species identification significantly improved when the in-house-built database was combined with the Bruker Filamentous Fungi Library compared with that for the Bruker library alone (Aspergillus, 93% versus 69%; Fusarium, 84% versus 42%; and Scedosporium, 94% versus 18%, respectively).
TEXT
Rapid, accurate mold identification is important due to the widening spectrum of pathogens and species-specific differences in antifungal susceptibility (1–3). Matrix-assisted laser desorption ionization−time of flight mass spectrometry (MALDI-TOF MS) has proven useful, but mold identification remains challenged by the limited access to validated purpose-built databases that are necessary because of small species and strain representations in commercial libraries (4–16).
Given the prior poor performance of the Bruker Filamentous Fungi Library v1.0 (Bruker Daltonics, Bremen, Germany) for mold identification using the manufacturer-recommended broth-based protein extraction methods (in our laboratory >50% of isolates were not identified; internal data) and because the geographic generalizability of in-house-built databases is not yet known, we hypothesized that a MS library of molds relevant to our region (17–21) will improve identification. Here, we constructed an in-house database containing 117 strains (see Table S1 in the supplemental material) covering 28 species of Aspergillus, Scedosporium, and Fusarium encountered in Australia. Challenge isolates (also n = 117; 21 species) comprising 55 Aspergillus, 45 Fusarium, and 17 Scedosporium clinical strains (Table 1) were then used to assess the performance of the Bruker library alone versus that of the Bruker library supplemented with the in-house library for species identification.
TABLE 1.
Performance according to genera and species of the Bruker library and the Bruker library supplemented with the customized in-house database when evaluated against a set of challenge clinical isolatesa
| Organism (no. isolates) | No. (%) isolates of its genus or species correctly identified by the specified log score value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bruker library alone |
Bruker library plus in-house database |
|||||||
| ≥2.00 | ≥1.70 | <1.70 (no ID) | Mis-ID | ≥2.00 | ≥1.70 | <1.70 (no ID) | Mis-ID | |
| Aspergillus spp. (55) | 38 (69) | 43 (78) | 8 (14.5) | 4 (7.2) | 51 (93) | 52 (95) | 2 (3.6) | 1 (1.8)b |
| Aspergillus alliaceus (2) | 0 (0) | 0 (0) | 2 (0) | 0 (0) | 2 (100) | 2 (100) | 0 (0) | 0 (0) |
| Aspergillus creber (2) | 0 (0) | 0 (0) | 0 (0) | 2 (100)b | 2 (100) | 2 (100) | 0 (0) | 0 (0) |
| Aspergillus flavus (5) | 5 (100) | 5 (100) | 0 (0) | 0 (0) | 5 (100) | 5 (100) | 0 (0) | 0 (0) |
| Aspergillus fumigatus (14)c | 14 (100) | 14 (100) | 0 (0) | 0 (0) | 14 (100) | 14 (100) | 0 (0) | 0 (0) |
| Aspergillus lentulus (3) | 0 (0) | 0 (0) | 3 (100) | 0 (0) | 3 (100) | 3 (100) | 0 (0) | 0 (0) |
| Aspergillus nidulans (6) | 6 (100) | 6 (100) | 0 (0) | 0 (0) | 6 (100) | 6 (100) | 0 (0) | 0 (0) |
| Aspergillus niger (9) | 7 (78) | 9 (100) | 0 (0) | 0 (0) | 8 (89) | 9 (100) | 0 (0) | 0 (0) |
| Aspergillus terreus (7) | 3 (43) | 6 (86) | 1 (14.2) | 0 (0) | 7 (100) | 7 (100) | 0 (0) | 0 (0) |
| Aspergillus versicolor (3) | 3 (100) | 3 (100) | 0 (0) | 0 (0) | 3 (100) | 3 (100) | 0 (0) | 0 (0) |
| Aspergillus viridinutans complex (2) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 0 (0) |
| Aspergillus sydowii (2) | 0 (0) | 0 (0) | 0 (0) | 2 (100)b | 1 (50) | 1 (50)b | 0 (0) | 1 (50)b |
| Fusarium spp. (45) | 19 (42) | 38 (84) | 7 (15.5) | 0 (0) | 38 (84) | 45 (100) | 1 (2.2) | 0 (0) |
| Fusarium chlamydosporum complex (4) | 0 (0) | 4 (100) | 0 (0) | 0 (0) | 2 (50) | 4 (100) | 0 (0) | 0 (0) |
| Fusarium dimerum complex (3) | 3 (100) | 3 (100) | 0 (0) | 0 (0) | 3 (100) | 3 (100) | 0 (0) | 0 (0) |
| Fusarium fujikuroi complex (13) | 10 (77) | 13 (100) | 0 (0) | 0 (0) | 13 (100) | 13 (100) | 0 (0) | 0 (0) |
| Fusarium incarnatum-equiseti complex (4) | 0 (0) | 2 (50) | 2 (50) | 0 (0) | 2 (50) | 4 (100) | 0 (0) | 0 (0) |
| Fusarium oxysporum complex (9) | 3 (33) | 7 (78) | 2 (22.2) | 0 (0) | 8 (89) | 9 (100) | 0 (0) | 0 (0) |
| Fusarium solani complex (12) | 6 (50) | 9 (75) | 3 (25) | 0 (0) | 10 (83) | 11 (92) | 1 (8.3) | 0 (0) |
| Scedosporium/Pseudallescheria spp. (17) | 3 (18) | 8 (47) | 3 (0) | 6 (35.3) | 16 (94) | 17 (100) | 0 (0) | 0 (0) |
| Scedosporium apiospermum (5) | 2 (40) | 3 (60) | 2 (40) | 0 (0) | 4 (80) | 5 (100) | 0 (0) | 0 (0) |
| Scedosporium aurantiacum (7) | 0 (0) | 1 (14) | 0 (0) | 6 (85.7)d | 7 (100) | 7 (100) | 0 (0) | 0 (0) |
| Scedosporium boydii (1) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 | 1 | 0 (0) | 0 (0) |
| Scedosporium prolificans (4) | 1 (25) | 3 (75) | 1 (25) | 0 (0) | 4 (100) | 4 (100) | 0 (0) | 0 (0) |
| Total (117) | 63 (54) | 89 (76) | 18 (15) | 10 (9) | 105 (90) | 113 (97) | 3 (3) | 1 (<1) |
n = 117. ID, identification; mis-ID, misidentification.
Misidentified as Aspergillus versicolor.
All A. fumigatus sensu stricto.
Misidentified as Scedosporium apiospermum.
All isolates were identified using phenotypic methods (22) with definitive identification by DNA sequencing of the internal transcribed spacer (ITS) (all isolates), β-tubulin (Aspergillus and Scedosporium spp.), and partial elongation factor-1alpha (EF-1α) (to identify Fusarium to the species complex level) gene regions (23–26). Sequence data were analyzed against the Centraalbureau voor Schimmelkultures (http://www.cbs.knaw.nl/Collections/BioloMICSSequences.aspx?file=all), International Society for Human and Animal Mycology ITS (http://its.mycologylab.org/), and Fusarium-ID (http://www.fusariumdb.org/index.php) databases, and species were assigned using published criteria (27).
Protein extraction for MALDI-TOF MS was performed as previously described (11). The Bruker bacterial test standard (Bruker Daltonics) was used for calibration and Aspergillus ustus CBS 261.67T scoring of ≥2.00 was required for quality extraction and spectra acceptability (11). The in-house database was constructed using published protocols (11, 28) with 20 to 25 quality spectra required for mass spectral profile (MSP) creation using default Biotyper settings (Bruker Daltonics).
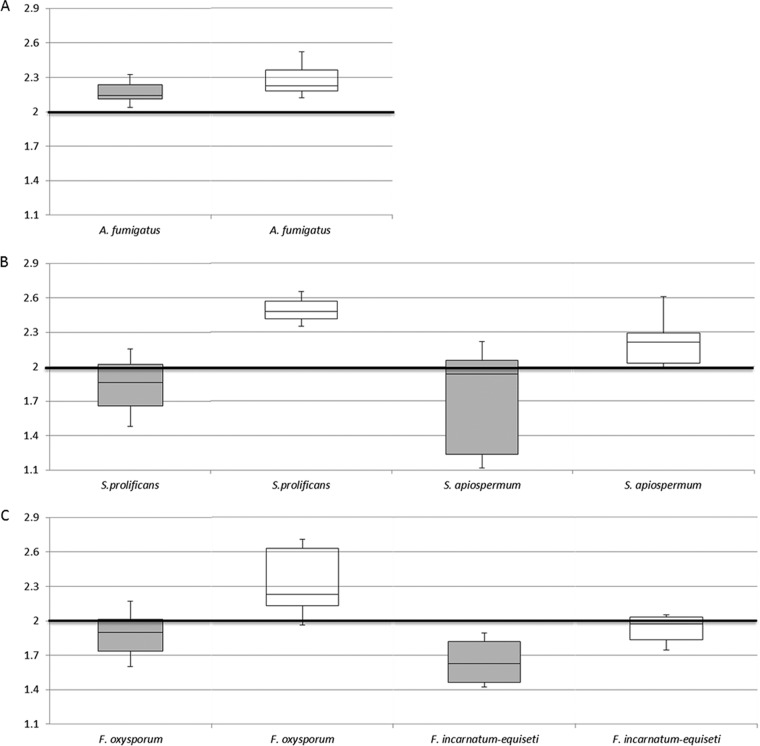
For challenge isolates, spectra were acquired in technical triplicates using established protocols (11) and queried against (i) the Bruker library and (ii) the Bruker library combined with the in-house database. Manufacturer-recommended cutoff values (for species, log score of ≥2.00; for genus, score of ≥1.70 to ≤1.99) were maintained. Median values and interquartile ranges (IQR) of log scores obtained by the two database sets were calculated for all isolates and then by genera and selected species using SPSS v21 software (SPSS Inc., Chicago, IL). The Wilcoxon signed-rank test was used to compare the median scores and Friedman's two-way analysis of variance to compare the distributions of scores (Fig. 1). McNemar's test was used to compare the frequencies of paired log score data at cutoffs of ≥2.00 versus ≥1.70 and ≥2.00 versus ≥1.80.
FIG 1.
Box and whisker plots illustrating the median mass spectral log scores and interquartile range of scores for Aspergillus fumigatus (A), Scedosporium prolificans and Scedosporium apiospermum (B) and Fusarium oxysporum and Fusarium incarnatum-equiseti complex (C). Scores achieved when challenged against the Bruker library alone are shown in gray shaded boxes, and those achieved by the combined Bruker library and in-house database are shown in the open boxes. The numbers on the y axis represent MALDI-TOF MS log scores.
Given the importance of protein extraction for acquiring quality spectra, we used a well-validated method (11) to develop our database. All isolate-specific spectra were reproducible and matched well with their corresponding MSP. Comparison of the extraction method used here with another proposed agar-based method (13) for spectra quality may be of clinical interest.
The Bruker library correctly identified 54% (63/117) of the challenge isolates to the species level and an additional 22% (26/117) to the genus level; all of the 18 isolates not identified were of species not represented in the library (Table 1). When the library was supplemented with the in-house database, identification improved significantly (at a level of α = 0.01) with 90% and 96.8% of isolates identified to the species and genus levels, respectively. The median of log scores of the supplemented Bruker library was significantly higher (α = 0.01) as was the distribution of scores for individual genera (selected species are represented in Fig. 1). Reductions in the IQRs of scores were evident except for Aspergillus fumigatus and Fusarium oxysporum. Various proportional increases in species identifications of molds after supplementation of commercial libraries with in-house-built databases have been reported (5–13); Schulthess et al. noted an increase from 52.4% to 79% after supplementation of the same Bruker library (v1.0) (28).
The Bruker library alone identified 69% (38/55) of Aspergillus isolates, including all A. fumigatus sensu stricto and Aspergillus flavus; however, 1/7 Aspergillus terreus (represented in the library) and most uncommon species, including Aspergillus lentulus (spectra not represented) were not identified (Table 1). The combined database identified 93% of the isolates to the species level except for one (50%) Aspergillus sydowii and both Aspergillus viridinutans strains. Failure to identify isolates or inaccurate identification (see below) resulted from the absence or limited number of relevant spectra in the Bruker database.
Notably, the largest (5-fold) improvement in identification after database supplementation was for Scedosporium (Table 1) with species identification for 94% (16/17) isolates, including all seven Scedosporium aurantiacum strains (versus 18% [3/17] by the Bruker library alone). Although Scedosporium prolificans is represented in the Bruker library, only 1/4 isolates was identified to the species level. Scedosporiosis is the second most common non-Aspergillus mold infection in Australia with 24% of infections due to S. aurantiacum (19, 21, 29). Adoption of our database in other Australian centers has the potential to remove reliance on molecular approaches to identify Scedosporium to the species level, overcoming the limitations of other dedicated Scedosporium databases that utilize different software, thus limiting their wider application (8, 12, 30).
The supplemented Bruker library also identified 2-fold as many Fusarium isolates to the species complex level (84% versus 42%; significant at α = 0.01) (Table 1). While the Bruker library performed well for the Fusarium dimerum complex, it identified only 33 to 50% of the Fusarium solani and Fusarium oxysporum complexes, the most common causes of fusariosis (31, 32). The combined database identified all but one F. solani strain, while two isolates each of Fusarium chlamydosporum and Fusarium incarnatum-equiseti complex had log scores between 1.7 and 1.99 but with correct identification. The challenge of acquiring reproducible spectra for Fusarium spp. is noteworthy (5, 9, 26); one study (6) identified only 1/6 F. solani complex when interrogated against the same Bruker library (v1.0).
While analyzing Fusarium isolates to the species complex level, rather than to the individual species level may be a limitation, higher species-level discrimination may be unnecessary because susceptibility differences between members of the same species complex do not appear to be clinically relevant (26, 33). Species delineation of Fusarium necessitates multilocus gene sequence typing (MLST) incorporating at least four loci, and different MLST schemes are recommended for different species complexes (3, 34). Triest et al. (26) built a database of 40 Fusarium species where species identification was achieved for 82.8% and 91% of isolates using log scores of ≥2.00 and ≥1.80, respectively, with 97% identified to the species complex level (versus 84% herein).
Lowering the log score cutoff to ≥1.80 for species identification significantly improved identification (69.2% versus 54% of isolates at ≥2.00; α = 0.01) by the Bruker library; this difference was significant also for Fusarium (42% to 73.3%; α = 0.01). While there was also improvement in proportional identification for the combined database, no statistical significance was demonstrated for any of the three genera at the cutoff of ≥1.80 (Fusarium; 84% to 95.6%; Aspergillus, 93% to 95%; and Scedosporium, 94% to 100%). Improvements in mold identification have been reported, including at cutoffs of ≥1.70 (26, 28) and as low as ≥1.40 using customized databases (12). The modest improvement in species identification observed in our study at a cutoff of ≥1.80 (and even at ≥1.70; data not shown) suggests that the representation of locally relevant spectra in a database, rather than lowering the threshold is more important for test performance.
Twelve isolates (one Scedosporium apiospermum, seven Fusarium, and four Aspergillus) (Table 1) were not identified to the species/species complex level by the combined database but eight had correct identifications at log scores of ≥1.70 to ≤2.00. Of 10 isolates called as misidentifications by the Bruker library, two each were Aspergillus creber and Aspergillus sydowii (misidentified as Aspergillus versicolor) and six were S. aurantiacum (as S. apiospermum). The combined library called one A. sydowii strain as A. versicolor. It is possible that identification errors exist in the Bruker database. While we “challenged” the combined database with only 21/28 species with in-house-built spectra, evaluation of other species is ongoing.
In summary, we have developed a clinically relevant database containing 28 species of Aspergillus, Fusarium, and Scedosporium. This library is portable across diagnostic laboratories within Australasia to supplement the Bruker library.
Supplementary Material
ACKNOWLEDGMENTS
We thank Wieland Meyer and Krystyna Maszewska for donating several mold isolates toward the study.
Funding Statement
A.F.L. was supported by the Intramural Research Program of the National Institutes of Health. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00906-16.
REFERENCES
- 1.Pfaller MA, Diekema DJ. 2010. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 2.Lackner M, de Hoog GS, Verweij PE, Najafzadeh MJ, Curfs-Breuker I, Klaassen CH, Meis JF. 2012. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother 56:2635–2642. doi: 10.1128/AAC.05910-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Xiao M, Kong F, Chen S, Dou HT, Sorrell T, Li TY, Xu YC. 2011. Accurate and practical identification of 20 Fusarium species by seven-locus sequence analysis and reverse line blot hybridisation, and an in vitro antifungal susceptibility study. J Clin Microbiol 49:1890–1898. doi: 10.1128/JCM.02415-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bader O. 2013. MALDI-TOF MS-based species identification and typing in medical mycology. Proteomics 13:788–799. doi: 10.1002/pmic.201200468. [DOI] [PubMed] [Google Scholar]
- 5.Iriart X, Lavergne RA, Fillaux J, Valentin A, Magnaval JF, Berry A, Cassaing S. 2012. Routine identification of medical fungi by the new Vitek MS matrix-assisted laser desorption ionization-time of flight system with a new time-effective strategy. J Clin Microbiol 50:2107–2110. doi: 10.1128/JCM.06713-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Liu Y, Teng S, Liao C, Hung C, Sheng W, Teng L, Hsueh P. 2015. Evaluation of the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry Bruker Biotype for identification of Penicillium marneffei, Paecilomyces, Fusarium solani, Rhizopus species and Pseudallescheria boydii. Front Microbiol 6:679. doi: 10.3389/fmicb.2015.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alanio A, Beretti JL, Dauphin B, Mellado E, Quesne G, Lacroix C, Amara A, Berche P, Nassif X, Bougnoux ME. 2011. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for fast and accurate identification of clinically relevant Aspergillus species. Clin Microbiol Infect 17:750–755. doi: 10.1111/j.1469-0691.2010.03323.x. [DOI] [PubMed] [Google Scholar]
- 8.Sitterlé E, Giraud S, Leto J, Bouchara JP, Rougeron A, Morio F, Dauphin B, Angebault C, Quesne G, Beretti J-L, Hassouni N, Nassif X, Bougnoux M. 2014. Matrix-assisted laser desorption ionization−time of flight mass spectrometry for fast and accurate identification of Pseudallescheria/Scedosporium species. Clin Microbiol Infect 20:929–935. doi: 10.1111/1469-0691.12574. [DOI] [PubMed] [Google Scholar]
- 9.Marinach-Patrice C, Lethuillier A, Marly A, Brossas J-Y, Gene J, Symoens F, Datry A, Guarro J, Mazier D, Hennequin C. 2009. Use of mass spectrometry to identify clinical Fusarium isolates. Clin Microbiol Infect 15:634–642. doi: 10.1111/j.1469-0691.2009.02758.x. [DOI] [PubMed] [Google Scholar]
- 10.De Carolis E, Posteraro B, Lass-Florl C, Vella A, Florio AR, Girmenia C, Colozza C, Tortorano AM, Sanguinetti M, Fadda G. 2012. Species identification of Aspergillus, Fusarium, and Mucorales with direct surface analysis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Microbiol Infect 18:475–484. doi: 10.1111/j.1469-0691.2011.03599.x. [DOI] [PubMed] [Google Scholar]
- 11.Lau A, Drake S, Calhoun L, Henderson C, Zelazny A. 2013. Development of a clinically comprehensive database and a simple procedure for identification of moulds from solid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 51:828–834. doi: 10.1128/JCM.02852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker P, de Bel A, Martiny D, Ranque S, Piarroux R, Cassagne C, Detandt M, Hendrickx M. 2014. Identification of filamentous fungi isolates by MALDI-TOF mass spectrometry: clinical evaluation of an extended reference spectra library. Med Mycol 52:826–834. doi: 10.1093/mmy/myu064. [DOI] [PubMed] [Google Scholar]
- 13.Cassagne C, Ranque S, Normand A-C, Fourquet P, Thiebault S, Planard C, Hendrickx M, Piarroux R. 2011. Mold routine identification in the clinical laboratory by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. PLoS One 6:e28425. doi: 10.1371/journal.pone.0028425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hettick J, Green B, Buskirk A, Kashon M, Slaven J, Janoka R, Blachere F, Schmechel D, Beezhold D. 2008. Discrimination of Aspergillus isolates at the species and strain level by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry fingerprinting. Anal Biochem 380:276–281. doi: 10.1016/j.ab.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 15.Gautier M, Ranque S, Normand A-C, Becker P, Packeu A, Cassagne C, Olivier CCL, Hendrickx M, Piarroux R. 2014. Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry: revolutionizing clinical laboratory diagnosis of mould infections. Clin Microbiol Infect 20:1366–1371. doi: 10.1111/1469-0691.12750. [DOI] [PubMed] [Google Scholar]
- 16.Normand A-C, Cassagne C, Ranque S, L'Olivier C, Fourquet P, Roesems S, Hendrickx M, Piarroux R. 2013. Assessment of various parameters to improve MALDI-TOF MS reference spectra libraries constructed for the routine identification of filamentous fungi. BMC Microbiol 13:76. doi: 10.1186/1471-2180-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lackner M, Klaassen CH, Meis J, Gerritsvan Den Ende G, De Hoog GS. 2012. Molecular identification tools for sibling species of Scedosporium and Pseudallescheria. Med Mycol 50:497–508. doi: 10.3109/13693786.2011.618939. [DOI] [PubMed] [Google Scholar]
- 18.Miceli MH, Lee SA. 2011. Emerging moulds: epidemiological trends and antifungal resistance. Mycoses 54:e666–e678. doi: 10.1111/j.1439-0507.2011.02032.x. [DOI] [PubMed] [Google Scholar]
- 19.Slavin M, Van Hal S, Sorrell TC, Lee A, Marriott DJ, Daveson K, Kennedy K, Hajkowicz K, Halliday C, Athan E, Bak N, Cheong E, Heath C, Morrissey CO, Kidd S, Beresford R, Blyth C, Korman TM, Robinson JO, Meyer W, Chen SC-A. 2015. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin Microbiol Infect 21:490e1–490e10. doi: 10.1016/j.cmi.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Morrissey CO, Chen SC, Sorrell TC, Milliken S, Bardy PG, Bradstock KF, Szer J, Halliday CL, Gilroy NM, Moore J, Schwarer AP, Guy S, Bajel A, Tramontana AR, Spelman T, Slavin MA, Australasian Leukaemia Lymphoma Group and the Australia and New Zealand Mycology Interest Group. 2013. Galactomannan and PCR-directed versus the standard culture and histological-based antifungal strategy for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect Dis 13:519–528. doi: 10.1016/S1473-3099(13)70076-8. [DOI] [PubMed] [Google Scholar]
- 21.Heath CH, Slavin MA, Sorrell TC, Handke R, Harun A, Philips M, Nguyen Q, Delhaes L, Ellis D, Meyer W, Chen SC, Australian Scedosporium Study Group. 2009. Population-based surveillance for scedosporiosis in Australia: epidemiology, disease manifestations and emergence of Scedosporium aurantiacum infection. Clin Microbiol Infect 15:689–693. doi: 10.1111/j.1469-0691.2009.02802.x. [DOI] [PubMed] [Google Scholar]
- 22.De Hoog GS, Guarro J, Gene J, Figueras MJ. 2000. Atlas of clinical fungi. The ultimate benchtop tool for diagnostics, version 4.1.2, 4th ed Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- 23.White T, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis M, Gelfand D, Sninsky J, White T (ed.), PCR protocols: a guide to methods and applications. Academic Press, New York, NY. [Google Scholar]
- 24.Yurkov A, Krueger D, Begerow D, Arnold N, Takka MT. 2012. Basidiomycetous yeasts from boletales fruiting bodies and their interactions with the mycoparasite Sepedonium chrysospermum and the host fungus Paxillus. Microb Ecol 63:295–303. doi: 10.1007/s00248-011-9923-7. [DOI] [PubMed] [Google Scholar]
- 25.Glass NL, Donaldson G. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triest D, Stubbe D, De Cremer K, Peirard D, Normand A-C, Piarroux R, Detandt M, Hendrickx M. 2015. Use of matrix-assisted laser desorption ionization−time of flight mass spectrometry for identification of molds of the Fusarium genus. J Clin Microbiol 53:465–476. doi: 10.1128/JCM.02213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2008. Interpretative criteria for identification of bacteria and fungi by DNA sequencing; approved guideline. CLSI document MM18-A Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.Schulthess B, Ledermann R, Mouttet F, Zbinden A, Bloemberg G, Bottger E, Hombach M. 2014. Use of the Bruker MALDI Biotyper for identification of moulds in the clinical mycology laboratory. J Clin Microbiol 52:2797–2803. doi: 10.1128/JCM.00049-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delhaes L, Arun A, Chen SC, Nguyen Q, Slavin M, Heath CH, Maszewska K, Halliday C, Robert V, Sorrell T, Australian Scedosporium (AUSCEDO) Study Group, Meyer W. 2008. Molecular typing of Australian Scedosporium isolates show genetic variability and numerous S. aurantiacum. Emerg Infect Dis 154:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulibaly O, Marinach-Patrice C, Cassagne C, Piarroux R, Mazier D, Ranque S. 2011. Pseudallescheria/Scedosporium complex species identification by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Med Mycol 49:621–626. doi: 10.3109/13693786.2011.555424. [DOI] [PubMed] [Google Scholar]
- 31.Nucci M, Anaissie E. 2007. Fusarium infections in immunocompromised patients. Clin Microbiol Rev 20:695–704. doi: 10.1128/CMR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Donnell K, Sutton DA, Rinaldi MG, Gueidan C, Crous PW, Geiser DM. 2009. Novel multilocus sequence typing scheme reveals high generic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J Clin Microbiol 47:3851–3861. doi: 10.1128/JCM.01616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tortorano AM, Prigitano A, Esposto M, Arsenijevic A, Kolarovic J, Ivanovic D, Paripovic L, Klingspor L, Norday I, Hamal P, Candoni A, Caira M, Drogari Apiranthitou M, ECMM Working Group. 2014. European Confederation of Medical Mycology (ECMM) epidemiological survey on invasive infections due to Fusarium species in Europe. Eur J Clin Microbiol Infect Dis 33:1623–1630. doi: 10.1007/s10096-014-2111-1. [DOI] [PubMed] [Google Scholar]
- 34.van Diepeningen A, Brankovics B, Iltes J, van der Lee T, Waalwijk C. 2015. Diagnosis of Fusarium infections: approaches to identification by the clinical mycology laboratory. Curr Fungal Infect Rep 9:135–143. doi: 10.1007/s12281-015-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.