Abstract
The Gram-positive anaerobic bacterium Clostridium perfringens is widely distributed in nature, especially in soil and the gastrointestinal tracts of humans and animals. C. perfringens causes gas gangrene and food poisoning, and it produces extracellular enzymes and toxins that are thought to act synergistically and contribute to its pathogenesis. A complicated regulatory network of toxin genes has been reported that includes a two-component system for regulatory RNA and cell-cell communication. It is necessary to clarify the global regulatory system of these genes in order to understand and treat the virulence of C. perfringens. We summarize the existing knowledge about the regulatory mechanisms here.
Keywords: C. perfringens, regulatory network
1. Introduction
Clostridium perfringens is a Gram-positive anaerobic spore-forming bacterium that is widely distributed in nature, especially in soil and the intestinal tracts of humans and animals. It causes clostridial myonecrosis (gas gangrene) and mild enterotoxemia in humans. C. perfringens is divided into five types (types A through E) (Table 1) depending on its major toxin (α-, β-, ε-, and ι-toxins) production [1,2,3]. The α-toxin (CPA) is conserved in all types of C. perfringens and the structural gene is located on the chromosome. Three other major toxins are encoded on the plasmid, and the classification of C. perfringens is based on the presence of plasmids encoding β-toxin (CPB, cpb gene), ε-toxin (ETX, etx gene), and ι-toxin (ITX, iap and ibp gene) [4]. Among the five types of C. perfringens, the type A strains are recognized as major pathogens in humans [3].
Table 1.
Classification of C. perfringens.
| Type | Toxins Produced | |||
|---|---|---|---|---|
| Alpha | Beta | Epsilon | Iota | |
| A | + | − | − | − |
| B | + | + | + | − |
| C | + | + | − | − |
| D | + | − | + | − |
| E | + | − | − | + |
C. perfringens produces a wide variety of enzymes and toxins. A genome analysis showed that C. perfringens cannot synthesize many types of amino acids because it lacks many genes related to amino acid biosynthesis [5]. The growth of C. perfringens in a host organism, therefore, requires both the degradation of host tissues in small-size nutrients and the rapid transport of the nutrients into bacterial cells. This ability of C. perfringens is essential for it to survive and grow in the host. Once C. perfringens starts growing, the host organism imports sugar compounds from host tissues that are degrading, and C. perfringens then uses the sugar to produce energy through an anaerobic glycolysis pathway. During this process, C. perfringens produces abundant gas and makes the conditions more suitable for further growth.
C. perfringens produces various toxins, and the production of toxins is tightly regulated by specific gene regulatory systems [6]. For instance, the production of Clostridium perfringens enterotoxin (CPE) by a C. perfringens type A strain occurs only when the bacterium sporulates. In addition, many toxin genes are regulated by the VirS/VirR two-component system and an accessory gene regulator (agr) quorum-sensing (QS) system. Several two-component systems and RNA regulators comprise a tight network to control the timing of the toxin production.
In this paper, we summarize the progress of the research related to the regulation of toxin production by C. perfringens.
2. The Regulation of Toxin Genes by Two-Component Regulatory Systems
Two-component systems (TCSs) are important regulatory systems that sense the composition of the environment and transmit the information into the cell [7]. Normally, a TCS consists of the membrane-bound sensor histidine kinase (which senses the environment or stimuli) and a cytoplasmic response regulator that acts as a transcriptional regulator. The sensor histidine kinase transfers a phosphate group to the response regulator and activates the response regulator. The activated response regulator regulates the expression of many genes [8].
There are 48 genes for two-component regulatory systems in the C. perfringens strain 13 genome [5]. Twenty-eight of these genes are sensor histidine kinase genes, and the other 20 genes are response regulators. Several sensor histidine kinases and response regulators are orphans, and there are no response regulators or histidine kinase genes next to them [5]. Several TCSs in C. perfringens have been extensively analyzed, and here we discuss three TCSs related to toxin gene regulation.
2.1. The Regulation of Toxin Genes by the VirS/VirR System
One of the most important and well-characterized TCSs in C. perfringens is the VirR/VirS system. The VirR/VirS system was originally identified in 1994 as a regulator for the α-toxin gene plc, the κ-toxin gene colA and the θ-toxin gene pfoA [9,10]. The VirS/VirR system consists of a gene for the response regulator virR and a gene for the sensor histidine kinase virS. VirS has a relatively hydrophobic N-terminus with six transmembrane regions. The autophosphorylation site is located at a putative cytoplasmic loop between the N-terminal transmembrane 4 and 5. Three domains that are conserved in other sensor histidine kinases are located in the C-terminal domain. The N-terminus of VirR has a conserved domain that needs to receive a phosphate group from cognate sensor histidine kinase [9,11].
Three toxin genes, plc, pfoA, and colA, are regulated by the VirS/VirR system, and the modes of their regulation are different. The pfoA gene has major and minor promoters, and only the major promoter is dependent on the VirS/VirR system. The, colA gene has two major promoters, and only one of them is VirR/VirS-dependent. plc has only one promoter, and it is dependent on the VirR/VirS system [10].
There is no common motif for VirR binding in the promoter region of these three toxin genes, and it is thus suspected that there is a secondary regulator under the VirS/VirR system that acts to regulate these genes. To identify novel genes that are regulated by the VirS/VirR system, a differential display method has been used. The analysis revealed vrr gene (which encodes VR-RNA, VirR regulated RNA), and a comparison of the promoter regions of pfoA and vrr showed a VirR-binding motif (CCAnTT(n = 15)CCAGTT(n = 3)Cac) [12,13].
An analysis of the complete genome sequence of C. perfringens strain 13 [5] showed that there are five genes that have a VirR-binding motif in their promoter region. These five genes are pfoA (Perfringolysin O or theta toxin gene), hyp7 (subsequently named vrr), virT (encoding a hypothetical protein gene), virU (encoding a hypothetical protein gene), and ccp (encoding alpha-clostripain) gene, and the expression of these five genes was suggested to be regulated by the VirS/VirR system [5]. VR-RNA, which is encoded by vrr, and VirT and VirU, which are encoded by virT and virU, respectively, have regulatory activity. These regulatory mechanisms are reviewed below in a later section. In a different strain, EHE-NE18, the VirR-binding motif was also identified in the promoter region of netB, a gene for the pore-forming toxin NetB [14]. NetB production is positively regulated by the VirR/VirS system in C. perfringens strain EHE-NE18 [14]. The binding motif was experimentally confirmed [13,15], and it was shown that the genes are regulated by the VirS/VirR system at the transcriptional level.
The VirS/VirR system is located on the chromosome, but it can regulate genes on both the chromosome and the plasmid. For example, collagen-adhesin gene (cna) and beta-2 toxin (cpb2), which are on plasmids in C. perfringens type A strain 13, are regulated by the VirS/VirR system. However, cna is negatively regulated, whereas cpb2 is positively regulated by this system [16]. C. perfringens strains are classified into types A–E depending on the production of major toxins. Except for α-toxin gene, the genes of the major C. perfringens toxins are located on the plasmid.
cpb encodes C. perfringens beta toxin (CPB), which is a major toxin produced by type B and type C strains that contribute to hemorrhagic necrotic enteritis. The production of CPB by a type C strain has been reported to be regulated by the VirS/VirR system [17]. CPB is required for the intestinal virulence of type C strains [18]. VirS/VirR contributes to the pathogenicity of type C strains through the regulation of CPB production [17]. These data indicated that the VirS/VirR system is a key regulator to control the genes on both the chromosome and the plasmid.
It was reported that the VirS/VirR system is also important for sensing cells [19]: when a type C strain of C. perfringens came into contact with Caco-2 cells, the toxin production was quickly up-regulated, whereas a VirR mutant could not induce the toxin production even when the strain came into contact with the same type of cells. These data indicated that the VirS/VirR system is important for sensing the environment (especially cells in the environment) and for up-regulating the toxin production [19].
C. perfringens lacks many genes related to amino acid biosynthesis and, thus, its ability to distinguish different aspects of the environment and to upregulate the production of toxins and enzymes is important to its growth. The upregulation of toxin production by contact with Caco-2 cells thus appears to be one of the responses when C. perfringens recognizes the host cells and prepares to degrade the host cells [20]. However, it is not yet known how C. perfringens recognizes Caco-2 cells.
The regulation of toxins by the VirS/VirR system is not the regulation of only their expression. Iota toxin is the major toxin produced by C. perfringens type E strains. Iota toxin is produced as an inactive form, and proteolysis by a protease is needed to activate this toxin. It was reported that the cleavage of immature protein is accomplished by a VirS/VirR-dependent protease [21]. These data indicated that the activity of the iota toxin is regulated by the VirS/VirR system through the regulation of the protease activity required for the proteolysis [21].
In addition, as discussed below, this system has a secondary regulator, VR-RNA. Through VR-RNA, the VirS/VirR-VR-RNA cascade controls the expression of various gene categories [22]. The VirS/VirR system is, thus, a global gene regulator and is one of the most important regulators in C. perfringens.
2.2. The RevR System
The well-characterized secondary TCS in C. perfringens is the RevR system. RevR is similar to the response regulators PhoB and YycF of other Gram-positive bacteria [23]. PhoB is a response regulator of a TCS of C. kluyveri [24] and YycF is a response regulator of Bacillus subtilis [25]. RevR is a putative orphan response regulator, and there is no histidine kinase gene upstream or downstream of the revR gene. In other bacteria, phoB and yycf have cognate histidine kinases. A homology search for those histidine kinases showed that CPE1757 is a candidate for the cognate revR histidine kinase [26]. However, the cognate histidine kinase for revR has not been analyzed.
RevR appears to be a classical response regulator with an N-terminal receiver domain and a C-terminal domain with a putative winged helix-turn-helix motif. RevR has a regulatory effect on virulence-related genes in a VirS/VirR-independent manner [26]. A microarray analysis showed that more than 100 genes, including virulence-related genes and VirS/VirR-regulated genes, are regulated by RevR [26]. Among the virulence-related genes, the hyaluronidase genes nagH and nagL are regulated positively, and the sialidase gene nanI and the α-clostripain gene ccp are regulated negatively by this system [26]. Importantly, a RevR mutant strain showed attenuated virulence compared to the wild-type strain in a mouse myonecrosis model [26]. These data showed that RevR is important for the pathogenesis of C. perfringens.
Both the VirS/VirR and RevR systems are important in the regulation of virulence in C. perfringens.
2.3. ReeS (Regulator of Extracellular Enzymes Sensor)
An orphan histidine kinase called ReeS (regulator of extracellular enzymes sensor) was identified and extensively analyzed [23]. At the amino acid sequence level, ReeS retains conserved sensor histidine kinase domains, and also putative RE and YYY domains, which are conserved in hybrid sensor kinases. However, there is no potential DNA binding motif in the protein. There is no gene related to a two-component signal transduction system in close proximity to ReeS. It is, thus, thought that ReeS works as an orphan histidine kinase.
ReeS positively regulates the transcription of sialidase genes including the major sialidase gene, nanI; the minor sialidase genes nanJ. nanI and nanJ are also regulated by the VirS/VirR system, as discussed above [22]. However, the gene regulation by ReeS is independent from the VirS/VirR system. ReeS does not affect the gene expression of pfoA, plc, or colA, which are regulated by the VirS/VirR system, as well as that of the hyaluronidase genes nagH and nagL, which are regulated by RevR. It has been reported that sialidase is important for the virulence of other bacteria, but animal experiments using a ReeS mutant of C. perfringens showed that ReeS did not affect the virulence even when the sialidase production in the ReeS mutant was reduced [23].
TCS systems that have been analyzed showed that the sialidase gene nanI is regulated by all of the TCS systems that have been identified to date, i.e., the VirS/VirR, RevR, and ReeS systems [22,23,26]. The production of sialidase and sialic acid degradation are, thus, likely to be necessary for C. perfringens to acquire nutrients and survive in the host.
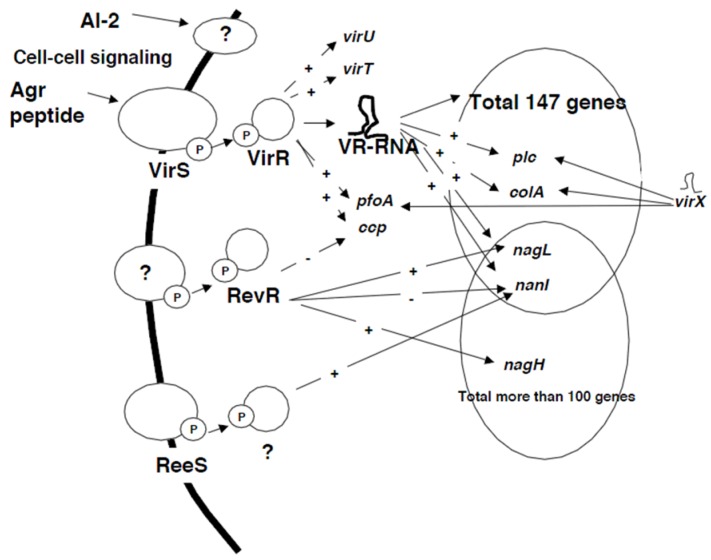
These TCSs seem to comprise a complex regulatory network of toxin genes (Figure 1). The identification of cognate histidine kinases or response regulators of the orphan TCSs and analyses of other putative TCSs are expected.
Figure 1.
The regulatory networks of C. perfringens type A strain 13. VirS/VirR-VR-RNA cascade regulates total of 147 genes including toxin genes. The response regulator RevR and sensor histidine kinase ReeS also regulate several toxin genes. These two-component regulatory systems develop a complex toxin gene regulatory network. +: positive regulation, −: negative regulation.
2.4. Regulation by RNA Molecules
Many small RNA molecules with regulatory functions have been reported in both prokaryotes and eukaryotes [27]. Indeed, genome analyses showed that >10% of RNA coding regions have a role in gene regulation; these regulatory RNAs regulate transcription and/or translation by binding the target mRNAs or proteins [28]. It was reported that small RNAs in several pathogenic bacteria regulate virulence-related genes [27,29,30]. In C. perfringens there are RNA molecules that act as transcriptional regulators. The mechanisms underlying the regulation of toxin genes by RNA molecules are discussed in this section.
2.5. VR-RNA
VR-RNA was the first RNA molecule reported to have regulatory function in Clostridia. This RNA is regulated by the VirS/VirR system, and it was thus named VR-RNA for “VirR regulated RNA” [12,13]. VR-RNA was originally identified in a screening for VirS/VirR-regulated genes, and it was first thought that the VirR-regulated gene was hyp7 gene (CPE0957). The mutant strain of this region showed a reduced amount of plc and colA transcripts. Further analysis showed that the region that is responsible for the regulation of plc and colA was not a protein-coding region of hyp7 and that the RNA, itself, has a regulatory activity; hyp7 was, therefore, renamed vrr. The region that is responsible for the regulatory activity is located in the small 3′-end region of the VR-RNA. A computer analysis of VR-RNA 386 nt from the transcription start site to the probable terminator region showed that the predicted structure is a stem-and-loop structure and that the 5′ and 3′ ends were predicted to pair [13].
VR-RNA was originally discovered as a secondary regulator of plc and colA in the regulation cascade of the VirS/VirR system. To identify the VirS/VirR-VR-RNA-regulated genes, microarray experiments were performed. The transcriptome data showed that 147 genes (30 single genes and 21 putative operons) including virulence-related genes are regulated by the VirR/VirS-VR-RNA cascade [22]. The VR-RNA-regulated virulence-related genes were not only plc and cola, but also sialidase genes (nanI and nanJ) and a hyaluronidase gene, nagL. In addition to virulence-related genes, the genes that are closely related to bacterial survival in the host tissue, including enzymes, transporters, and related genes for energy metabolism, are regulated by the VirS/VirR-VR-RNA cascade. These data indicated that the VirS/VirR-VR-RNA system is a global regulatory system that is needed to coordinate multiple functions of genes so that the C. perfringens can multiply in the host which would, in turn, cause the typical symptoms of gas gangrene.
The mechanisms underlying the regulation by VR-RNA of 147 genes in total [22] are not yet known. It is likely that other factors, e.g., VR-RNA binding proteins or third regulatory factors, exist in this system.
2.6. VirT and VirU
A genome sequence analysis of the C. perfringens strain 13 genome revealed five genes that have a VirR-binding motif [5]. Three of these five genes were demonstrated to regulate toxin genes as RNA regulators. One of the three genes is VR-RNA, discussed above. The other two genes are virT and virU. virT is located upstream of ccp (alpha-clostripain gene), which also has a VirR-binding motif. virT and virU transcription are strongly and positively regulated by VirR/VirS [31]. An analysis of virT mutant strain TS190 showed that VirT has a negative effect on the expressions of pfoA and ccp. pfoA and ccp have VirR-binding motifs in their promoter region, but VirT does not have a regulatory effect on vrr or virU, which also have a VirR-binding motif [31].
VirU, another RNA regulator, also affects toxin gene expression. A virU mutant has not been constructed, but an over-expression experiment showed that VirU regulates pfoA, vrr, ccp, and virT positively. VirT does not regulate the expression of plc or colA, whereas VirU had a slight effect on the expressions of colA and plc [31].
The regulation of toxin genes by VirT and VirU is not as strong as the regulation by VR-RNA, but VirT and VirU may fine-tune the transcription of virulence-related genes [31]. The entire regulatory system mediated by VirT and VirU is not yet clear, and broad-scale analyses are needed to address this topic.
2.7. VirX
VirX is another important RNA regulator in C. perfringens strain 13. VirX is an RNA transcribed from the CPE0646 region. It regulates plc, colA, and pfoA transcription in a VirS/VirR-independent manner [32]. virX gene encodes a 51-amino acid polypeptide, but it was shown that the RNA itself of this region has a regulatory effect on toxin genes. VR-RNA does not regulate pfoA transcription, but VirX positively regulates pfoA transcription. Thereby, pfoA is positively regulated by VirX at the mid-exponential phase, whereas colA and plc are positively regulated in the late-exponential phase [32]. The regulation of toxin genes is not so strong, but it was demonstrated that VirX is a negative regulator of sporulation [33]. It was thus suggested that between control/balance sporulation and toxin production is needed.
Since the enterotoxin gene cpe is expressed only when C. perfringens sporulates, the regulation of cpe by VirX has been analyzed, and the results showed that VirX regulates cpe transcription negatively in the sporulating condition.
3. Regulation of Toxin Genes by Cell-Cell Communication
Cell-cell communication is an important procedure used by bacteria to share information and, thus, ”talk“ to each other. Quorum sensing is the process that controls cell-to-cell communication. Bacteria sense the cell density or concentration of a signal substance. Once the concentration of signal substance reaches a threshold, this triggers gene regulation [34,35]. Several studies reported that the virulence factors of pathogenic bacteria are regulated by a quorum-sensing system [36,37,38].
Several cell-cell communication systems have been reported in many bacteria. Cell-cell communication in Gram-positive bacteria is mediated by two types of systems. The first system uses a peptide as a signal molecule to stimulate gene expression. This system, called an agr system, has been well studied in Staphylococcus aureus [39]. The second system is called the luxS system, which was first identified in Vibrio harveyi [40]. The luxS system is common to Gram-positive and Gram-negative bacteria and, thus, this system has been thought to be a tool that bacteria can use to communicate among each other beyond their own species.
Clostridium perfringens has both an agr quorum sensing system and a luxS system. The role of cell-to-cell communication in toxin gene expression is discussed below.
3.1. The Autoinducer 2 (AI-2) System
In the C. perfringens strain 13 genome, there is a luxS gene that is responsible for the production of AI-2 (autoinducer 2), which is the signal substance of quorum-sensing in both Gram-positive and Gram-negative bacteria. The AI-2 system was first observed in Vibrio harveyi as a quorum-sensing signal to stimulate its luminescence [40]. The culture supernatant of C. perfringens strain 13 stimulated the luminescence of V. harveyi strain, BB170, but the luxS mutant of C. perfringens strain 13 could not stimulate the luminescence [41]. These results showed that the luxS gene is responsible for the production of a signal substance that stimulates the luminescence of V. harveyi, AI-2.
The role of the luxS gene in C. perfringens was examined, and the authors reported that luxS positively regulates plc, colA and pfoA at the transcriptional level [41]. The transcription of plc and colA was reduced at the early log-phase, but pfoA transcription was reduced at the early to late-log phase in the luxS mutant [41]. The luxS mutant showed a recovered pfoA transcription level with the addition of wild-type supernatant or the supernatant of Escherichia coli DH5α carrying the luxS gene of C. perfringens. These data clearly indicated that the luxS gene is responsible for the production of AI-2 and that AI-2 regulates the toxin gene expression in C. perfringens.
As noted above, the AI-2 system is a common system in Gram-positive and Gram-negative bacteria [42]. Almost one-half of all bacteria the genome sequence of which is present in the database have the luxS gene [43]. It has, thus, been speculated that AI-2 is needed by bacteria that have an AI-2 system in order to communicate with each other beyond the species.
The importance of normal flora has been revealed, and AI-2 might be an important factor for maintaining the balance of the normal flora and pathogenic bacteria. Moreover, because it has been reported that AI-2 regulates many virulence factors, luxS is considered a therapeutic target in infectious diseases [44]. Further detailed analyses can be expected to contribute new research data in this area.
3.2. The agr System
The agr (accessory gene regulator) system is a well-characterized cell-cell communication system in Gram-positive bacteria, especially in S. aureus [45,46]. It is an important system that regulates virulence genes in a quorum-sensing manner in S. aureus and many other Gram-positive pathogenic bacteria [47,48,49]. In S. aureus, autoinducer propeptide (AIP) is produced from the agrD gene, and then AgrB is required to modify the AgrD propeptide. There is a two-component system, agrAC, downstream of agrBD. AgrC is a sensor protein for AgrD peptide, and once the concentration of AIP reaches a threshold, AgrC is activated. The activated AgrC then activates its cognate response regulator AgrA. Subsequently, activated AgrA regulates the RNA regulator RNAIII located upstream of the agr operon.
RNAIII regulates the transcription of various virulence genes in S. aureus. A homologous gene of S. aureus, agrBD was identified in the C. perfringens strain 13 genome, and the amino acid sequence showed that there is a conserved cysteine residue, which is important for the formation of a thiolactone ring, in the AgrD of C. perfringens. However, there is no TCS upstream or downstream of agrBD in the C. perfringens strain 13 genome.
A mutant strain of agrBD, TS230, showed weak hemolysis on a blood agar plate [50]. However, TS230 recovered hemolysis when the strain was cross-streaked with TS133 (a VirSR mutant that is hemolysis-negative, but produces a signal substance) from just after the crossing point [50]. This finding indicates that strain TS230 lost the signal substance to stimulate θ-toxin (or PFO) production, but if TS230 receives the signal from another strain, it can recover the toxin production. There are several studies from the 1970s that concern toxin production by C. perfringens [51,52,53,54]. Those studies describe two types of strains that were θ-toxin-negative even though they had a θ-toxin gene. One type of these strains cannot recover the toxin production by culture with other toxin-negative strains, whereas the other type can recover the toxin production [51]. In light of those findings, it has been thought that there must be a signal substance to stimulate θ-toxin production in C. perfringens. It seems that the agr system is closely related to this phenomenon.
The mutant strain of agrBD, TS230, reduced the transcription of plc, colA, and pfoA and recovered the transcription by complementation of agrBD gene [50], indicating that the agr system in C. perfringens regulates at least plc, colA, and pfoA genes among the virulence-related genes. In another report using the non-foodborne human gastrointestinal disease strain F5603, it has been shown that agrB regulates the production of CPE and CPB2 positively [55]. Moreover, the supernatant of the wild-type strain or complemented strain can stimulate the transcription toxin genes in TS230 [50]. These data indicated that the agrBD region of C. perfringens is responsible for the production of a signal substance.
An experiment using a agrBD-virSR double-mutant strain showed that the supernatant of wild-type C. perfringens strain 13 could not stimulate the toxin gene expression [50], suggesting that VirS is one of the sensor proteins for the signal peptide. Although a genome analysis showed that there is no TCS system around the agrBD gene as discussed above, these data indicated that VirSR corresponds to AgrAC of S. aureus. In addition, in S. aureus, RNAIII is a key factor of the agr system and AgrAC regulates RNAIII transcription; RNAIII then regulates the expression of many genes [39]. The manner of gene regulation by RNAIII seems to correspond to that of the VR-RNA of C. perfringens. In S. aureus, regulatory genes are clustered in the genome, but in C. perfringens, virS/virR, VR-RNA and agrBD are scattered in the genome.
The signal peptide that stimulates gene expression is produced from agrD. In S. aureus, amino acid sequences of AgrD are classified into four groups depending on the amino acid sequences [56]. In contrast, there is no such sequence variation in the C. perfringens AgrD amino acid sequence.
In other toxin-type C. perfringens strains, an agr system has been reported to have an important role in toxin production (Table 2). The five C. perfringens types depend on the production of major toxins. The agrB null mutant showed less CPB production and recovered the production by complementation with agrB in C. perfringens type B strains CN1793 and CN1795; however, the productions of epsilon toxin (ETX) and CPB2 were not affected by agrB mutation [57]. Experiments with type C strain CN3685 also showed that CPB production is regulated by the agr system [58]. It was also shown that the agr system is required to cause necrotizing enteritis by C. perfringens type B strain CN3685 [58].
Table 2.
Regulation of toxin production or toxin gene expression in C. perfringens.
| Type | Toxin (gene) | Regulation by VirR/VirS Ref: [9,10,14,16,17,22,57] | Regulation by agr Ref: [50,55,57,58,59] | VR-RNA Ref: [12,13,22] | VirT Ref: [31] | VirU Ref: [31] | VirX Ref: [32] | CodY Ref: [60] | ReeS Ref: [23] | RevR Ref: [26] |
|---|---|---|---|---|---|---|---|---|---|---|
| A | CPA (plc) | Yes (Yes) | Yes (Yes) | (Yes) | No | No | (Yes) | NR | No | No |
| PFO (pfoA) | Yes (Yes) | Yes (Yes) | No | (Yes) | (Yes) | (Yes) | NR | No | No | |
| collagenase (colA) | (Yes) | (Yes) | (Yes) | No | No | (Yes) | NR | No | No | |
| sialidase (nanI) | Yes | NR | (Yes) | NR | NR | NR | NR | (Yes) | (Yes) | |
| Hyaluronidase (nagH) | (Yes) | NR | (Yes) | NR | NR | NR | NR | No | (Yes) | |
| Hyaluronidase (nagL) | (Yes) | NR | (Yes) | NR | NR | NR | NR | No | (Yes) | |
| α-clostripain (ccp) | (Yes) | NR | No | (Yes) | (Yes) | NR | NR | No | (Yes) | |
| CPE (cpe) | NR | Yes | NR | NR | NR | NR | NR | No | No | |
| NetB (netB) | Yes | NR | NR | NR | NR | NR | NR | No | No | |
| CPB2 (cpb2) | Yes | Yes | (Yes) | NR | NR | NR | NR | No | NR | |
| B | CPA (plc) | NR | Yes | NR | NR | NR | NR | NR | NR | NR |
| PFO (pfoA) | NR | Yes | NR | NR | NR | NR | NR | NR | NR | |
| CPB (cpb) | NR | Yes | NR | NR | NR | NR | NR | NR | NR | |
| ETX (etx) | NR | No | NR | NR | NR | NR | NR | NR | NR | |
| CPB2 (cpb2) | NR | No | NR | NR | NR | NR | NR | NR | NR | |
| C | CPA (plc) | Yes | Yes | NR | NR | NR | NR | NR | NR | NR |
| PFO (pfoA) | Yes | Yes | NR | NR | NR | NR | NR | NR | NR | |
| CPB (cpb) | Yes | Yes | NR | NR | NR | NR | NR | NR | NR | |
| D | CPA (plc) | Yes | Yes | NR | NR | NR | NR | No | NR | NR |
| PFO (pfoA) | Yes | Yes | NR | NR | NR | NR | No | NR | NR | |
| ETX (etx) | No | Yes(Yes) | NR | NR | NR | NR | Yes | NR | NR | |
| E | ITX (iap and ibp) | NR (for activity Yes) | NR | NR | NR | NR | NR | NR | NR | NR |
NR: not reported, Yes or No: regulation of toxin production level is reported, (Yes or No): regulation of gene expression is reported.
CPB is produced by type B and type C strains, and the above findings showed that the agr system could regulate the CPB production in both types of strains. In type D strain CN3718 too, the agr system has an important role in the strain’s virulence. Type D strains produce ETX, which is a pore-forming toxin considered the major virulence factor of type B and D strains. ETX production has been reported to be regulated by the agr system [59]. ETX production in a type B strain was not affected by the agr system, but in a type D strain it was affected by the agr system [59].
There are some differences in regulatory systems among the strains even when they have the same regulator and toxin genes [57,59]. Interestingly, the signal that is produced from agrD has been thought to be a signal for VirS. However, in the regulation of ETX production in type D strain CN3718, ETX production was regulated by the agr system, but not by the VirS/VirR system. This was a first report showing that the agr system does not always activate the VirS/VirR system [59].
Overall, the agr system has a crucial role in the virulence factor production and pathogenesis in C. perfringens.
4. Other Types of Regulation
CodY
Several regulatory proteins that are common in Gram-positive bacteria and related to metabolism have been reported. CodY protein is one such protein. CodY is thought to be involved in the adaptive response of Gram-positive bacteria to their environment. CodY senses the nutrient conditions of bacteria by binding GTP or branched-chain amino acid (BCAA) in the cell [61]. When the amount of GTP or BCAA is sufficient, CodY binds to the promoter region of the regulated genes in complex with GTP or BCAA. In the stationary phase, there are less nutrients, and CodY is not in complex with GTP or BCAA and has decreased affinity for the binding region of target genes.
It was reported that in low G + C Gram-positive bacteria, CodY regulates several virulence-related genes [62,63]. For instance, the production of toxin A and toxin B are regulated by CodY in C. difficile [64]. The function of CodY in C. perfringens type D strain CN3718 was investigated, and the analysis of a CodY mutant showed that CodY did not affect the production of PFO (θ-toxin) or PLC (α-toxin), but it positively regulated the production of ETX (ε-toxin) [60]. In contrast, the production of sialidase was negatively affected by CodY, mainly by the regulation of NanH.
The regulation of ETX occurs by CodY protein binding to the promoter region of the etx gene. A gel mobility shift assay showed that the CodY binding box is located between 21 and 354 bp upstream of the etx start codon [60]. However, the relationship between GTP and CodY or the nutrient condition has not been identified. In other bacteria, it has been reported there is a palindromic binding motif in the promoter region of regulated genes, and approx. Five percent of the genes in the genome are regulated by CodY [65]. It is, thus, likely that in C. perfringens, too, CodY has a global regulatory function. Additional studies are needed to elucidate the regulatory system involving CodY.
5. Conclusions
There are complex toxin gene regulatory systems in C. perfringens (Figure 1). Here we focused on the regulatory network of toxin genes, but the same network, i.e., the VirS/VirR-VR-RNA cascade, regulates various categories of genes, including extracellular enzymes, transporters, and genes for energy metabolism. The VirS/VirR-VR-RNA cascade is a global regulator that may control multiple cellular functions that enable C. perfringens to survive and multiply in infectious conditions. The VirS/VirR-VR-cascade is just one example; other regulators also regulate multifunction genes.
It may be difficult for C. perfringens to obtain nutrients from its host under infectious conditions, but it must get the nutrients to survive and multiply by degrading host tissue. The networks that have been demonstrated to regulate toxin genes would be required to coordinate the expression of genes that are needed to degrade the host tissue into small-size nutrients. This process turns into the necrotic infection of C. perfringens.
Many regulatory systems of toxin genes have been identified, but compared with other pathogenic bacteria we still have limited knowledge about the regulation of virulence. In other pathogenic clostridia, e.g., C. difficile and C. botulinum, it has been reported that certain amino acids have an effect on toxin production. The repression of toxin production by a mixture of specific amino acids is mediated by CodY. The presence of glucose represses the toxin production through CcpA [66]. The importance of alternative sigma factors for the regulation of toxin production has also been reported in clostridia, especially in C. botulinum and C. difficile [66,67]. Similar mechanisms to control the toxin production might exist in C. perfringens. Compared with C. difficile, less is known about the regulation of toxins by metabolites or alternative sigma factors in C. perfringens. More extensive research on the regulatory mechanisms of virulence of C. perfringens is highly desired. The elucidation of the detailed regulatory network of toxin production could enable the development of molecular-based preventive and therapeutic techniques for combatting C. perfringens infections and infectious diseases overall.
Acknowledgments
The work carried out in our laboratory was supported by KAKENHI (a Grant-in-Aid for Scientific Research) on Priority Areas “Applied Genomics” from the Ministry of Education, Culture, Sports, Science (17019021, 17790284, 22390081, 15390140), and Technology of Japan, and by Health and Labor Sciences Research Grants (Research on Food Safety). Tohru Shimizu passed away in February 2014. Tohru devoted his energy to figuring out the regulatory network of C. perfringens, and we thank him for his many years of support and guidance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hatheway C.L. Toxigenic clostridia. Clin. Microbiol. Rev. 1990;3:66–98. doi: 10.1128/CMR.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petit L., Gibert M., Popoff M.R. Clostridium perfringens: Toxinotype and genotype. Trends Microbiol. 1999;7:104–110. doi: 10.1016/S0966-842X(98)01430-9. [DOI] [PubMed] [Google Scholar]
- 3.Rood J.I. Virulence genes of Costridium perfringens. Annu. Rev. Microbiol. 1998;52:333–360. doi: 10.1146/annurev.micro.52.1.333. [DOI] [PubMed] [Google Scholar]
- 4.Hassan K.A., Elbourne L.D., Tetu S.G., Melville S.B., Rood J.I., Paulsen I.T. Genomic analyses of Clostridium perfringens isolates from five toxinotypes. Res. Microbiol. 2015;166:255–263. doi: 10.1016/j.resmic.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu T., Ohtani K., Hirakawa H., Ohshima K., Yamashita A., Shiba T., Ogasawara N., Hattori M., Kuhara S., Hayashi H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohtani K., Shimizu T. Regulation of toxin gene expression in Clostridium perfringens. Res. Microbiol. 2015;166:280–289. doi: 10.1016/j.resmic.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Wanner B.L. Is cross regulation by phosphorylation of two-component respomse regulator proteins important in bacteria? J. Bacteriol. 1992;174:2053–2058. doi: 10.1128/jb.174.7.2053-2058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziejman M., Mekalanos J.J. Two-component signal transduction and its role in the expression of bacterial virulance factors. In: Hoch J.A., Silhavy T.J., editors. Two-Component Signal Transduction. American Society for Microbiology; Washington, WA, USA: 1995. pp. 305–317. [Google Scholar]
- 9.Shimizu T., Ba-Thein W., Tamaki M., Hayashi H. The virR gene, a member of a class of two-component response regulators, regulates the production of the perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J. Bacteriol. 1994;176:1616–1623. doi: 10.1128/jb.176.6.1616-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ba-Thein W., Lyristis M., Ohtani K., Nisbet I.T., Hayashi H., Rood J.I., Shimizu T. The virr/virs locus regulates the transcription of genes encoding extracellular toxin production in Clostridium perfringens. J. Bactreriol. 1996;178:2514–2520. doi: 10.1128/jb.178.9.2514-2520.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyristis M., Bryant A.E., Sloan J., Awad M.M., Nisbet I.T., Stevens D.L., Rood J.I. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol. Microbiol. 1994;12:761–777. doi: 10.1111/j.1365-2958.1994.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 12.Banu S., Ohtani K., Yaguchi H., Swe T., Cole S.T., Hayashi H., Shimizu T. Identification of novel virr/virs-regulated genes in Clostridium perfringens. Mol. Microbol. 2000;35:854–864. doi: 10.1046/j.1365-2958.2000.01760.x. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu T., Yaguchi H., Ohtani K., Banu S., Hayashi H. Clostridial VirR/VirS regulon involves a regulatory RNA molecule for expression of toxins. Mol. Microbiol. 2002;43:257–265. doi: 10.1046/j.1365-2958.2002.02743.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheung J.K., Keyburn A.L., Carter G.P., Lanckriet A.L., Van Immerseel F., Moore R.J., Rood J.I. The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens. Infect. Immun. 2010;78:3064–3072. doi: 10.1128/IAI.00123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung J.K., Dupuy B., Deveson D.S., Rood J.I. The spatial organization of the virr boxes is critical for virr-mediated expression of the perfringolysin O gene, pfoA, from Clostridium perfringens. J. Bacteriol. 2004;186:3321–3330. doi: 10.1128/JB.186.11.3321-3330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohtani K., Kawsar H.I., Okumura K., Hayashi H., Shimizu T. The virr/virs regulatory cascade affects transcription of plasmid-encoded putative virulence genes in Clostridium perfringens. FEMS Microbiol. Lett. 2003;222:137–141. doi: 10.1016/S0378-1097(03)00255-6. [DOI] [PubMed] [Google Scholar]
- 17.Ma M., Vidal J., Saputo J., McClane B.A., Uzal F. The virs/virr two-component system regulates the anaerobic cytotoxicity, intestinal pathogenicity, and enterotoxemic lethality of Clostridium perfringens type C isolate CN3685. mBio. 2011;2:e00338-10. doi: 10.1128/mBio.00338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayeed S., Uzal F.A., Fisher D.J., Saputo J., Vidal J.E., Chen Y., Gupta P., Rood J.I., McClane B.A. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol. Microbiol. 2008;67:15–30. doi: 10.1111/j.1365-2958.2007.06007.x. [DOI] [PubMed] [Google Scholar]
- 19.Vidal J.E., Ohtani K., Shimizu T., McClane B.A. Contact with enterocyte-like caco-2 cells induces rapid upregulation of toxin production by clostridium perfringens type C isolates. Cell Microbiol. 2009;11:1306–1328. doi: 10.1111/j.1462-5822.2009.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J., Ma M., Uzal F.A., McClane B.A. Host cell-induced signaling causes Clostridium perfringens to upregulate production of toxins important for intestinal infections. Gut Microbes. 2014;5:1–12. doi: 10.4161/gmic.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibert M., Petit L., Raffestin S., Okabe A., Popoff M.R. Clostridium perfringens iota-toxin requires activation of both binding and enzymatic components for cytopathic activity. Infect. Immun. 2000;68:3848–3853. doi: 10.1128/IAI.68.7.3848-3853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohtani K., Hirakawa H., Tashiro K., Yoshizawa S., Kuhara S., Shimizu T. Identification of a two-component VirR/VirS regulon in Clostridium perfringens. Anaerobe. 2010;16:258–264. doi: 10.1016/j.anaerobe.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Hiscox T.J., Harrison P.F., Chakravorty A., Choo J.M., Ohtani K., Shimizu T., Cheung J.K., Rood J.I. Regulation of sialidase production in Clostridium perfringens by the orphan sensor histidine kinase rees. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamarche M.G., Wanner B.L., Crepin S., Harel J. The phosphate regulon and bacterial virulence: A regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 2008;32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- 25.Winkler M.E., Hoch J.A. Essentiality, bypass, and targeting of the YycFG (VicRK) two-component regulatory system in gram-positive bacteria. J. Bacteriol. 2008;190:2645–2648. doi: 10.1128/JB.01682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiscox T.J., Chakravorty A., Choo J.M., Ohtani K., Shimizu T., Cheung J.K., Rood J.I. Regulation of virulence by the RevrR response regulator in Clostridium perfringens. Infect. Immun. 2011;79:2145–2153. doi: 10.1128/IAI.00060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson J., Cossart P. RNA-mediated control of virulence gene expression in bacterial pathogens. Trends Microbiol. 2003;11:280–285. doi: 10.1016/S0966-842X(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 28.Romby P., Charpentier E. An overview of RNAs with regulatory functions in gram-positive bacteria. Cell Mol. Life Sci. 2009;67:217–237. doi: 10.1007/s00018-009-0162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geissmann T., Possedko M., Huntzinger E., Fechter P., Ehresmann C., Romby P. Regulatory RNAs as mediators of virulence gene expression in bacteria. Handb. Exp. Pharmacol. 2006;137:9–43. doi: 10.1007/3-540-27262-3_2. [DOI] [PubMed] [Google Scholar]
- 30.Romby P., Vandenesch F., Wagner E.G. The role of RNAs in the regulation of virulence-gene expression. Curr. Opin. Microbiol. 2006;9:229–236. doi: 10.1016/j.mib.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Okumura K., Ohtani K., Hayashi H., Shimizu T. Characterization of genes regulated directly by the virr/virs system in Clostridium perfringens. J. Bacteriol. 2008;190:7719–7727. doi: 10.1128/JB.01573-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohtani K., Bhowmik S.K., Hayashi H., Shimizu T. Identification of a novel locus that regulates expression of toxin genes in Clostridium perfringens. FEMS Microbiol. Lett. 2002;209:109–114. doi: 10.1016/S0378-1097(02)00493-7. [DOI] [PubMed] [Google Scholar]
- 33.Ohtani K., Hirakawa H., Paredes-Sabja D., Tashiro K., Kuhara S., Sarker M.R., Shimizu T. Unique regulatory mechanism of sporulation and enterotoxin production in Clostridium perfringens. J. Bacteriol. 2013;195:2931–2936. doi: 10.1128/JB.02152-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller M.B., Bassler B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 35.Bassler B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999;2:582–587. doi: 10.1016/S1369-5274(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 36.Rutherford S.T., Bassler B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold. Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waters C.M., Bassler B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev Bio.l. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 38.Bassler B.L. Small talk. Cell-to-cell communication in bacteria. Cell. 2002;109:421–424. doi: 10.1016/S0092-8674(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 39.Lyon G.J., Novick R.P. Peptide signaling in Staphylococcus aureus and other gram-positive bacteria. Peptides. 2004;25:1389–1403. doi: 10.1016/j.peptides.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Schauder S., Shokat K., Surette M.G., Bassler B.L. The luxs family of bacterial autoinducers: Biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 41.Ohtani K., Hayashi H., Shimizu T. The luxs gene is involved in cell-cell signaling for toxin production in Clostridium perfringens. Mol. Microbiol. 2002;44:171–179. doi: 10.1046/j.1365-2958.2002.02863.x. [DOI] [PubMed] [Google Scholar]
- 42.Xavier K.B., Bassler B.L. Luxs quorum sensing: More than just a numbers game. Curr. Opin. Microbiol. 2003;6:191–197. doi: 10.1016/S1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 43.Federle M.J. Autoinducer-2-based chemical communication in bacteria: Complexities of interspecies signaling. Contrib. Microbiol. 2009;16:18–32. doi: 10.1159/000219371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belizario J.E., Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balaban N., Novick R.P. Autocrine regulation of toxin synthesis by Staphylococcus aureus. Proc. Natl. Acad. Sci. USA. 1995;92:1619–1623. doi: 10.1073/pnas.92.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novick R.P., Muir T.W. Virulence gene regulation by peptides in Staphylococci and other gram-positive bacteria. Curr. Opin. Microbiol. 1999;2:40–45. doi: 10.1016/S1369-5274(99)80007-1. [DOI] [PubMed] [Google Scholar]
- 47.Peng H.-L., Novic R.P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 1988;170:4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.George E.A., Muir T.W. Molecular mechanisms of agr quorum sensing in virulent staphylococci. Chembiochem. 2007;8:847–855. doi: 10.1002/cbic.200700023. [DOI] [PubMed] [Google Scholar]
- 49.Autret N., Raynaud C., Dubail I., Berche P., Charbit A. Identification of the agr locus of listeria monocytogenes: Role in bacterial virulence. Infect. Immun. 2003;71:4463–4471. doi: 10.1128/IAI.71.8.4463-4471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohtani K., Yuan Y., Hassan S., Wang R., Wang Y., Shimizu T. Virulence gene regulation by the agr system in Clostridium perfringens. J. Bacteriol. 2009;191:3919–3927. doi: 10.1128/JB.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higashi Y., Chazono M., Inoue K., Yanagase Y., Amano T., Shimada K. Complementation of theta toxinogenecity between mutants of two groups of Clostridium perfringens. Biken. J. 1973;16:1–9. [PubMed] [Google Scholar]
- 52.Imagawa T., Tatsuki T., Higashi Y., Amano T. Complementation characteristics of newly isolated mutants from two groups of strains of Clostridium perfringens. Biken J. 1981;24:13–21. [PubMed] [Google Scholar]
- 53.Imagawa T., Higashi Y. An activity which restores theta toxin activity in some theta toxin-deficient mutants of Clostridium perfringens. Microbiol. Immunol. 1992;36:523–527. doi: 10.1111/j.1348-0421.1992.tb02050.x. [DOI] [PubMed] [Google Scholar]
- 54.Rood J.I., Wilkinson R.G. Isolation and characterization of Clostridium perfringens mutants altered in broth hemagglutinin and sialidase production. J. Bacteriol. 1975;123:419–427. doi: 10.1128/jb.123.2.419-427.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J., Chen J., Vidal J.E., McClane B.A. The agr-like quorum-sensing system regulates sporulation and production of enterotoxin and beta2 toxin by Clostridium perfringens type A non-food-borne human gastrointestinal disease strain F5603. Infect. Immun. 2011;79:2451–2459. doi: 10.1128/IAI.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novick R.P. Autoinduction and signal transduction in the regulation of Staphylococcal virulence. Mol. Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 57.Chen J., McClane B.A. Role of the agr-like quorum-sensing system in regulating toxin production by Clostridium perfringens type B strains cn1793 and cn1795. Infect. Immun. 2012;80:3008–3017. doi: 10.1128/IAI.00438-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vidal J.E., Ma M., Saputo J., Garcia J., Uzal F.A., McClane B.A. Evidence that the agr-like quorum sensing system regulates the toxin production, cytotoxicity and pathogenicity of Clostridium perfringens type C isolate CN3685. Mol. Microbiol. 2012;83:179–194. doi: 10.1111/j.1365-2958.2011.07925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J., Rood J.I., McClane B.A. Epsilon-toxin production by Clostridium perfringens type D strain CN3718 is dependent upon the agr operon but not the VirS/VirR two-component regulatory system. mBio. 2011;2 doi: 10.1128/mBio.00275-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Li J., Ma M., Sarker M.R., McClane B.A. CodY is a global regulator of virulence-associated properties for Clostridium perfringens type D strain CN3718. mBio. 2013;4 doi: 10.1128/mBio.00770-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stenz L., Francois P., Whiteson K., Wolz C., Linder P., Schrenzel J. The CodY pleiotropic repressor controls virulence in gram-positive pathogens. FEMS Immunol. Med. Microbiol. 2011;62:123–139. doi: 10.1111/j.1574-695X.2011.00812.x. [DOI] [PubMed] [Google Scholar]
- 62.Chateau A., van Schaik W., Joseph P., Handke L.D., McBride S.M., Smeets F.M., Sonenshein A.L., Fouet A. Identification of CodY targets in Bacillus anthracis by genome-wide in vitro binding analysis. J. Bacteriol. 2013;195:1204–1213. doi: 10.1128/JB.02041-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Schaik W., Chateau A., Dillies M.A., Coppee J.Y., Sonenshein A.L., Fouet A. The global regulator CodY regulates toxin gene expression in Bacillus anthracis and is required for full virulence. Infect. Immun. 2009;77:4437–4445. doi: 10.1128/IAI.00716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dineen S.S., Villapakkam A.C., Nordman J.T., Sonenshein A.L. Repression of Clostridium difficile toxin gene expression by CodY. Mol. Microbiol. 2007;66:206–219. doi: 10.1111/j.1365-2958.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 65.Belitsky B.R., Sonenshein A.L. Genome-wide identification of Bacillus subtilis CodY-binding sites at single-nucleotide resolution. Proc. Natl. Acad. Sci. USA. 2013;110:7026–7031. doi: 10.1073/pnas.1300428110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bouillaut L., Dubois T., Sonenshein A.L., Dupuy B. Integration of metabolism and virulence in Clostridium difficile. Res. Microbiol. 2015;166:375–383. doi: 10.1016/j.resmic.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connan C., Popoff M.R. Two-component systems and toxinogenesis regulation in Clostridium botulinum. Res. Microbiol. 2015;166:332–343. doi: 10.1016/j.resmic.2014.12.012. [DOI] [PubMed] [Google Scholar]